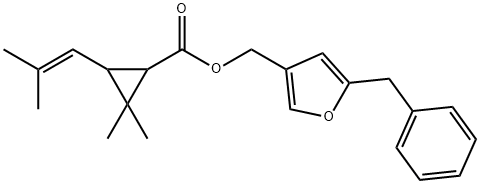
RESMETHRIN
- CAS No.
- 10453-86-8
- Chemical Name:
- RESMETHRIN
- Synonyms
- Seco;ari-b;Syntox;xylate;Vectrin;CHRYSON;For-syn;Scourge;Premgard;SBP 1382
- CBNumber:
- CB9311222
- Molecular Formula:
- C22H26O3
- Molecular Weight:
- 338.44
- MOL File:
- 10453-86-8.mol
- MSDS File:
- SDS
- Modify Date:
- 2023/9/20 8:34:21
| Melting point | 56.5℃ |
|---|---|
| Boiling point | bp >180° (dec) |
| Density | 0.96 g/cm3 |
| vapor pressure | ﹤l×10-5 Pa (25 °C) |
| refractive index | 1.5287 (20℃) |
| Flash point | closed cup: 264.2°F (129°C) |
| storage temp. | 0-6°C |
| solubility | Chloroform (Slightly), Methanol (Slightly, Heated) |
| Water Solubility | 0.038 mg l-1 (25 °C) |
| BRN | 1626510 |
| EPA Substance Registry System | Resmethrin (10453-86-8) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS07,GHS09 |
|---|---|
| Signal word | Warning |
| Hazard statements | H302-H410 |
| Precautionary statements | P264-P270-P273-P301+P312-P391-P501 |
| Hazard Codes | Xn,N |
| Risk Statements | 22-50/53 |
| Safety Statements | 60/61-61-60 |
| RIDADR | 3082 |
| WGK Germany | 3 |
| RTECS | GZ1310000 |
| HazardClass | 9 |
| PackingGroup | III |
| HS Code | 29321900 |
| Toxicity | LC50 (96 hr) in rainbow trout, bluegill sunfish (mg/l): 3.14, 7.2 (Rand) |
RESMETHRIN Chemical Properties,Uses,Production
Chemical Properties
Off-white to tan waxy solid or colorless crys- tals. Chrysanthemum-like odor. Commercial products may be dissolved in flammable organic solvents
Uses
Resmethrin is used to control a wide range of insects in horticultural, household, public health and animal health situations. It has some agricultural use but this is limited. The more active 1Rtrans form has a similar range of uses and is also used in stored grain products.
General Description
Colorless crystals or waxy solid. Insoluble in water. Used as an insecticide.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
A pyrethroid derivative.
Agricultural Uses
Insecticide: Resmethrin is currently used for mosquito control (by aerial application) in the USA, and may can also be used in greenhoused to control white fly. Resmethrin is a synthetic Type I pyrethroid insecticide registered for control of insects in residential, commercial and industrial settings, and in animal living areas. Resmethrin is also registered for use in food-handling establishments and as a restricted use pesticide when used in ULV spray to control adult mosquitoes in the interest of public health[83]. Restricted Use Pesticide (RUP) when formulated for use in mosquito abatement and pest control treatments at nonagricultural sites. Restricted due to extreme fish toxicity. Not approved for use in EU countries.
Trade name
(d-trans-isomer); SBP® 1382 (d-trans-isomer); d-transSBP® 1382 (d-trans-isomer); SBP®-1390; S. B. PENICK 1382®; SCOURGE®; SUN-BUGGER®; SYNTHRIN®; SYNTOX®; VECTRIN®; WHITMIRE® PT-110
Safety Profile
Poison by ingestion, andintravenous routes. Moderately toxic by inhalation andskin contact. When heated to decomposition it emits acridand irritating fumes.
Potential Exposure
Resmethrin is pyrethroid insecticide used for mosquito control (by aerial application) in the USA, and may can also be used in greenhouses to control white fly. Resmethrin is a synthetic Type I pyrethroid insecticide registered for control of insects in residential, commercial, and industrial settings, and in animal living areas. It is also registered for use in food handling estab- lishments and as a restricted use pesticide when used in ULV spray to control adult mosquitoes in the interest of public health . A United States Restricted Use Pesticide (RUP) when formulated for use in mosquito abatement and pest control treatments at nonagricultural sites. Restricted due to extreme fish toxicity.
Metabolic pathway
14C-resmethrin are individually administered orally to white leghorn hens, and more than 90% of the radioactivity is eliminated in the excreta within 24 h of treatment. The metabolic routes for both resmethrin isomers arise from ester hydrolysis and oxidation of the hydrolytic products. Some of these metabolites are further conjugated with glucuronic acid, sulfate, or other unidentified compounds before excretion.
Shipping
UN3352 Pyrethroid pesticide, liquid toxic, Hazard Class: 6.1; Labels: 6.1-Poisonous materials. UN3349 Pyrethroid pesticide, solid toxic, Hazard Class: 6.1; Labels: 6.1-Poisonous material
Incompatibilities
Decomposed by air, light, alkaline media, and temperatures .175℃. May react violently with strong oxidizers, bromine, 90% hydrogen peroxide, phos- phorus trichloride, silver powders or dust. Incompatible with silver compounds. Mixture with some silver compounds forms explosive salts of silver oxalate.
Waste Disposal
Incineration would be an effective disposal procedure where permitted. If an efficient incinerator is not available, the product should be mixed with large amounts of combustible material and contact with the smoke should be avoided. In accordance with 40CFR165. Follow recommendations for the disposal of pesticides and pesticide containers . Bury in noncrop land away from water. It would be better to mix the prod- uct with lime. Incineration would be an effective disposal procedure where permitted. If an efficient incinerator is not available, the product should be mixed with large amount of combustible material. Recommendable methods: Hydrolysis, landfill, incineration, and open burning. Not recommendable method: Discharge to sewer. Mix with saw- dust and burn at a remote place .
RESMETHRIN Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| Suprabhat Associates Private Limited | 08066085876Ext 652 | Delhi, India | 1 | 58 | Inquiry |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | China | 39916 | 58 | Inquiry |
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 | China | 12460 | 58 | Inquiry |
| Hebei Yanxi Chemical Co., Ltd. | +86-17531190177; +8617531190177 | China | 6011 | 58 | Inquiry |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | United States | 19892 | 58 | Inquiry |
| Hubei Ipure Biology Co., Ltd | +8613367258412 | China | 10326 | 58 | Inquiry |
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418671 | China | 34571 | 58 | Inquiry |
| AFINE CHEMICALS LIMITED | +86-0571-85134551 | China | 15395 | 58 | Inquiry |
| Shaanxi Didu New Materials Co. Ltd | +86-89586680 +86-13289823923 | China | 9003 | 58 | Inquiry |
10453-86-8(RESMETHRIN)Related Search:
1of4
chevron_right




