Barium sulfite
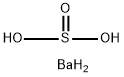
- CAS No.
- 7787-39-5
- Chemical Name:
- Barium sulfite
- Synonyms
- barium sulphite;BARIUM SULFITE;Sulfurous acid barium salt
- CBNumber:
- CB9407907
- Molecular Formula:
- BaH4O3S
- Molecular Weight:
- 221.42
- MOL File:
- 7787-39-5.mol
- MSDS File:
- SDS
- Modify Date:
- 2023/10/17 17:11:53
| Melting point | decomposed by heat [HAW93] |
|---|---|
| Density | 4.440 |
| solubility | insoluble in ethanol |
| form | white monoclinic crystals |
| color | white monoclinic crystals, crystalline |
| Water Solubility | 0.0197g/100g H2O (20°C); 0.0018g/100g (80°C); soluble dilute HCl [KIR78] [HAW93] |
| Solubility Product Constant (Ksp) | pKsp: 9.3 |
Barium sulfite price More Price(2)
Barium sulfite Chemical Properties,Uses,Production
Description
Barium Sulfite can be
prepared by reacting sodiumsulfite with bariumchloride:
BaCl2+ Na2SO3→BaSO3+ 2NaCl
Barium Sulfite occurs as white,
monoclinic crystals with a density of 4.44 g/cm3. Its
solubility in water is low (0.001125 g/100 ml) and it is
insoluble in ethanol. When heated, it decomposes at
480°C to the oxide and SO2 gas.
It has been used as a weighting material on oil-drilling
rigs to prevent “blow out” during drilling operations.
This salt is used for such purposes, presumably
because of its ability to absorb SO2 and H2S gases trapped in the rocks as drilling proceeds. The demand for barium sulfite is low.
Barium sulfite is available in limited quantities,
commercially.
Chemical Properties
White powder, decomposed by heat. Soluble in dilute hydrochloric acid; insoluble in water.
Uses
In paper manufacture.
Preparation
Barium sulfite is produced by the reaction of barium hydroxide, water and sulfur dioxide, or by the reaction of barium hydroxide and potassium bisulfite.
Barium sulfite Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Otto Chemie Pvt. Ltd. | +91 9820041841 | Mumbai, India | 5873 | 58 | Inquiry |
| Central Drug House(P) Ltd. | 91-11-49404040 | New Delhi, India | 6160 | 58 | Inquiry |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | China | 21669 | 55 | Inquiry |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | China | 39916 | 58 | Inquiry |
| Shaanxi Didu New Materials Co. Ltd | +86-89586680 +86-13289823923 | China | 9003 | 58 | Inquiry |
| Shaanxi DIDU pharmaceutical and Chemical Co., Ltd | 17691182729 18161915376 | China | 10011 | 58 | Inquiry |
| Hebei Zhenjia new material Co., LTD | 0319-5925599 13315915972 | China | 2917 | 58 | Inquiry |
| MP Biomedicals, Inc. | 949 833 2500 | United States | 6566 | 81 | Inquiry |
| City Chemicals Corporation | 800-248-2436 | United States | 6462 | 72 | Inquiry |
| Aithaca Chemical Corp. | (516) 229-2330 | United States | 1684 | 30 | Inquiry |
7787-39-5(Barium sulfite)Related Search:
1of4
chevron_right




