COBALT(II) IODIDE
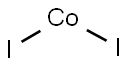
- CAS No.
- 15238-00-3
- Chemical Name:
- COBALT(II) IODIDE
- Synonyms
- COBALT IODIDE;cobalt diiodide;Einecs 239-283-2;COBALTOUS IODIDE;COBALT(II) IODIDE;cobaltiodide(coi2);Cobalt(II) diiodide;Cobaltiodideanhydrous;Cobalt(Ⅱ) iodide dihydrate;cobalt(ii) iodide, ultra dry
- CBNumber:
- CB9706215
- Molecular Formula:
- CoI2
- Molecular Weight:
- 312.74
- MOL File:
- 15238-00-3.mol
- Modify Date:
- 2024/5/29 14:23:27
| Melting point | >515 °C(lit.) |
|---|---|
| Boiling point | 570°C |
| Density | 5.45 g/mL at 25 °C(lit.) |
| Flash point | 570°C vac. |
| solubility | acetone: slightly soluble(lit.) |
| form | powder |
| color | White |
| Specific Gravity | 5.68 |
| Water Solubility | Soluble in water. |
| Sensitive | Hygroscopic |
| Merck | 14,2443 |
| Exposure limits | ACGIH: TWA 0.02 mg/m3 |
| Stability | hygroscopic |
| CAS DataBase Reference | 15238-00-3(CAS DataBase Reference) |
| EPA Substance Registry System | Cobalt iodide (CoI2) (15238-00-3) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS08,GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302+H312+H332-H315-H319-H335-H317-H341 | |||||||||
| Precautionary statements | P261-P280-P305+P351+P338-P201-P308+P313-P405-P501a | |||||||||
| Hazard Codes | Xn | |||||||||
| Risk Statements | 20/21/22-36/37/38 | |||||||||
| Safety Statements | 26-36 | |||||||||
| WGK Germany | 3 | |||||||||
| TSCA | Yes | |||||||||
| NFPA 704 |
|
COBALT(II) IODIDE price More Price(2)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| ALFA India | ALF-023119-22 | Cobalt(II) iodide, anhydrous, 99.5% (metals basis) | 15238-00-3 | 100g | ₹93291 | 2022-05-26 | Buy |
| ALFA India | ALF-023119-14 | Cobalt(II) iodide, anhydrous, 99.5% (metals basis) | 15238-00-3 | 25g | ₹20286 | 2022-05-26 | Buy |
COBALT(II) IODIDE Chemical Properties,Uses,Production
Description
Cobalt iodide appears as black crystals with a slight ‘iodine’-like odour. When heated to decomposition, it may emit toxic fumes of iodine and oxides of nitrogen. Anhydrous cobalt (II) iodide is sometimes used to test for the presence of water in various solvents. Cobalt (II) iodide is used as a catalyst, for example, in carbonylations. It catalyses the reaction of diketene with Grignard reagents, useful for the synthesis of terpenoids.
Chemical Properties
Brownish red crystalline powder
Physical properties
Exists in two isomorphous forms, α- and ?-forms; both modifications highly hygroscopic. The α-form is black hexagonal crystal; density 5.58 g/cm3; turns dark green in air; melts at 560°C; disolves in water giving pink coloration. The α-forms sublimes in vacuo, partly forming an isomorous yellow modification-the anhydrous β-form.
The β-modification is a yellow powder; density 5.45 g/cm3; converts to the α-form when heated to 400°C; absorbs moisture from air, the yellow powder becoming green droplets; dissolves readily in water forming a colorless solution which turns pink on heating.
The hexahydrate is red hexagonal crystals; density 2.90 g/cm3; loses water at 130°C giving anhydrous iodide; soluble in water, ethanol, acetone, chloroform and ether, forming colored solutions, (while the aqueous solution is red below 20°C and green above this temperature; the salt forms blue solution in ethanol, chloroform and ether).
Uses
Cobalt(II) iodide is used as a moisture and humidity indicator. It is also used as a catalyst.
Preparation
Cobalt(II) iodide is prepared by heating cobalt powder in a stream of hydrogen iodide at 400 to 450°C:
Co + 2HI → CoI2 + H2
The product obtained is the black crystalline α-form.
Cobalt(II) iodide also may be made by heating cobalt powder with iodine vapor.
Structure and conformation
Cobalt(II) iodide crystallizes in two polymorphs, the α- and β-forms. The α-polymorph comprises black hexagonal crystals, which turn dark green when exposed to air. Under vacuum at 500℃, samples of α-CoI2 sublime, forming the β-polymorph as yellow crystals. β-CoI2 also easily absorbs moisture from the air, converting into green hydrate. Below 400℃, β-CoI2 reverts back to the α-form. The anhydrous salt has the cadmium halide structure. The hexaaquo salt comprises separated [Co(H2O)6] 21 and iodide ions.
COBALT(II) IODIDE Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| S.K. CHEMICAL INDUSTRIES | +91-22-66370299 Ext 21 / 91-22-23437380 Ext 25 | New Delhi, India | 262 | 30 | Inquiry |
| Alfa Aesar | 1 800 209 7001 | Maharashtra, India | 6913 | 58 | Inquiry |
| Triveni chemicals | 08048762458 | New Delhi, India | 6093 | 58 | Inquiry |
| Ultra Pure Lab Chem Industries LLP | 08046077962 | Mumbai, India | 252 | 58 | Inquiry |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | China | 39916 | 58 | Inquiry |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-89586680 +86-18192503167 | China | 9030 | 58 | Inquiry |
| Henan Alfa Chemical Co., Ltd | +8618339805032 | China | 13141 | 58 | Inquiry |
| ShanDong Look Chemical Co.,Ltd. | +8617653113219 | China | 2739 | 58 | Inquiry |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503259 +86-19930503259 | China | 18435 | 58 | Inquiry |
| LEAPCHEM CO., LTD. | +86-852-30606658 | China | 43348 | 58 | Inquiry |





