キシレン 化学特性,用途語,生産方法
外観
無色澄明の液体ハーゼン10以下
定義
本品は、次の化学式で表される芳香属化合物である。
溶解性
水に不溶, エタノール, エーテルに混和。エタノール、エーテル、アセトン、二硫化炭素に可溶。水にほとんど不溶。エタノール及びジエチルエーテルに極めて溶けやすく、水にほとんど溶けない。
解説
キシレン,石油改質油中に大量に含まれる.また,石炭タール中にも存在する.o-,m-,およびp-キシレンの3種類の異性体があり,それぞれの沸点は接近しているので,これらを蒸留によって分離するには高度の精密蒸留が必要である.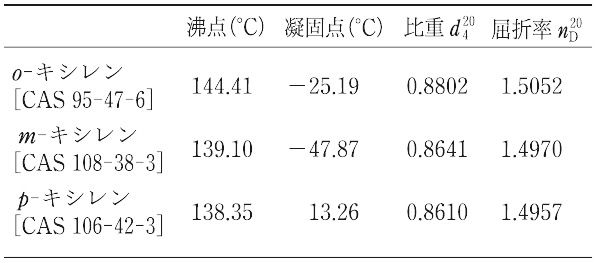 工業的には,石油改質油から芳香族炭化水素を溶剤により抽出し,キシレン混合物の精密蒸留によってo-キシレンおよびエチルベンゼンを分け,-60~-70 ℃ に冷却してp-キシレンを結晶化させて大部分を分けとる方法,混合キシレンからゼオライトによりp-キシレンを選択的に吸着分離する方法などが行われている.3種類のキシレンの間には熱力学的な平衡があり,触媒を使ってある程度これらの間に異性化を行わせることが可能である.各キシレンは酸化してベンゼンジカルボン酸(フタル酸,イソフタル酸およびテレフタル酸)にかえ,合成樹脂や合成繊維の原料に用いられるが,とくにo-およびp-キシレンは需要が多い.また,キシレン混合物は,工業的に各種の溶剤として使われる.
工業的には,石油改質油から芳香族炭化水素を溶剤により抽出し,キシレン混合物の精密蒸留によってo-キシレンおよびエチルベンゼンを分け,-60~-70 ℃ に冷却してp-キシレンを結晶化させて大部分を分けとる方法,混合キシレンからゼオライトによりp-キシレンを選択的に吸着分離する方法などが行われている.3種類のキシレンの間には熱力学的な平衡があり,触媒を使ってある程度これらの間に異性化を行わせることが可能である.各キシレンは酸化してベンゼンジカルボン酸(フタル酸,イソフタル酸およびテレフタル酸)にかえ,合成樹脂や合成繊維の原料に用いられるが,とくにo-およびp-キシレンは需要が多い.また,キシレン混合物は,工業的に各種の溶剤として使われる.
用途
病理組織標本作製用中間剤・透徹剤
用途
悪臭物質の試験におけるGC分析の標準液。
化粧品の成分用途
溶剤、香料
主な用途/役割
業務用の溶剤型接着剤、エマルション系接着剤に希に使用される。
使用上の注意
不活性ガス封入
説明
Xylene is used as a solvent. In this application, the mixture of isomers is often referred to
as xylenes or xylol. Solvent xylene often contains a small percentage of ethylbenzene. Like
the individual isomers, the mixture is colourless, sweet smelling, and highly flammable.
Application of xylene is extensive and includes, but is not limited to, printing, rubber, and
leather industries.
Similarly, it is used as a cleaning agent for steel and silicon wafers. In the petroleum
industry, xylene is also a frequent component of paraffin solvents, used when the tubing
becomes clogged with paraffin wax. Xylene is incompatible with strong oxidisers and is
known to cause fires and explosions. There are three forms of xylene in which the methyl
groups vary on the benzene ring: (i) meta-xylene, (ii) ortho-xylene, and (iii) para-xylene.
These forms are referred to as isomers. Xylene is a colourless, sweet-smelling liquid.
Xylene occurs naturally in petroleum and coal tar. Chemical industries produce xylene
from petroleum. It is also used as a cleaning agent and a thinner for paint and in paints,
in glues, in printing inks, and in varnishes. Xylene evaporates quickly from the soil and
surface water into the air.
化学的特性
Also known as dimethylbenzene, C6H4(CH3)2 is an isomeric mixture of 0- m-, and p-xylene. It is a clear liquid with various grades having different boiling points, that is insoluble in water and soluble in alcohol and ether,and used in aviation gasoline, coatings, lacquers, rubber cements, organic synthesis, and polyester resin manufacture.
物理的性質
Xylene is benzene to which two methyl groups have been added to two carbon atoms in the benzene ring. The addition of two methyl groups gives three isomers of xylene labeled according to the relative positions of the methyl groups. Ortho-xylene has methyl groups on consecutive carbons in the ring, meta-xylene's metyl groups are separated by a single carbon bonded to hydrogen atoms, and para-xylene has the methyl groups on carbon atoms on opposite sides of the ring. The three xylene isomers are abbreviated using o-,m-, p- for ortho, meta, and para, respectively. Xylene is used both as a mixture, where it is referred to as xylenes or xylol, and as individual isomers. Because their boiling points are close, separation using distillation is difficult. Therefore isomers are separated using techniques such as recrystallization and adsorption. Xylenes are flammable, colorless liquids with a pleasant odor. Xylene was first isolated from coal tar in the mid-19th century. The name xylene comes from the Greek word for wood xulon because xylene was obtained from the distillation of wood in the absence of oxygen.
使用
Xylene is used as a chemical feedstock in the chemical industry. Xylenes can undergooxidation where the side methyl groups are oxidized to give a carboxyl group (COOH)yielding a carboxylic acid. The particular acid produced depends on the isomer oxidized. Wheno-xylene is oxidized phthalic acid is produced, and when p-xylene is oxidized terephthalic acidresults. Terephthalic acid is one of the main feedstocks in making polyesters.Terephthalic acid reacts with ethylene glycol to form the ester polyethylene terephthalate(PET). PET is one of the most common plastics used as food and beverage containers. PETcontainers contain the recycling symbol with a number 1. PET is marketed using a numberof commercial names; the most generic of these is polyester. It is also the material known asDacron. Mylar is PET in the form of thin films. Although all three isomers of xylene are usedas chemical feedstocks, the greatest demand is for para-xylene to produce terephthalic acid.The smallest demand is for meta-xylene. Approximately 30 million tons of xylenes are usedannually worldwide.
定義
An organic
hydrocarbon present in the light-oil
fraction of crude oil. It is used extensively
as a solvent. There are three isomeric compounds
with this name and formula,
distinguished as 1,2-, 1,3-, and 1,4-dimethylbenzene
according to the positions
of the methyl groups on the benzene ring.
調製方法
Xylene is produced by catalytic reforming, and, depending
on the feedstock, yields of >85% can be achieved.
Commercially, xylene is also recovered from coal tar, yielding
a typical mixture of about 10–20% ortho, 40–70%
meta, and 10–25% para isomer. Impurities include ethylbenzene,
benzene, toluene, phenol, thiophene, and pyridine
(53, 438).
一般的な説明
A light colored to colorless liquid with a hydrocarbon odor. Flash point between 52 - 93°F. Less dense than water. Vapors are heavier than air. Vapors may irritate the eyes, nose, throat and respiratory tract. High vapor concentrations may cause central nervous system depression or damage. Liquid contact may irritate eyes and skin. Prolonged liquid contact mat result in defatting and drying of the skin. Avoid ingestion.
空気と水の反応
Highly flammable. Water insoluble.
反応プロフィール
Vigorous reactions, sometimes amounting to explosions, can result from the contact between these materials and strong oxidizing agents. They can react exothermically with bases and with diazo compounds. Substitution at the benzene nucleus occurs by halogenation (acid catalyst), nitration, sulfonation, and the Friedel-Crafts reaction.
健康ハザード
Exposures to xylene cause toxicity and adverse health effects to animals and humans. Acute and chronic exposure to xylene induces adverse effects on the skin and respiratory system of animals and humans. Prolonged exposure to xylene demonstrated burning effect, drying, defatting of skin, eye irritation, lung congestion, CNS excitation, depression, mucosal hemorrhage, and mild liver damage
安全性プロファイル
Moderately toxic by
intraperitoneal and subcutaneous routes.
LWdly toxic by ingestion and inhalation. An
experimental teratogen. Human systemic
effects by inhalation: olfactory changes,
conjunctiva irritation, and pulmonary
changes. Experimental reproductive effects.
Mutation data reported. A human eye
irritant, An experimental skin and severe eye
irritant. Some temporary corneal effects are
noted, as well as some conjunctival irritation
by instillation (adding drops to the eyes one
drop at a time). Irritation can start @ 200
ppm. A very dangerous fire hazard when
exposed to heat or flame; can react with
oxidzing materials. To fight fire, use foam,
CO2, dry chemical. When heated to
decomposition it emits acrid smoke and
irritating fumes. See also other xylene
entries.
発がん性
Mixed xylene and the individual xylene
isomers have tested negative in a wide variety
of genotoxic assays; they are considered to be
nonmutagenic. The IARC has determined that there is
inadequate evidence in humans and experimental
animals for the carcinogenicity of xylenes.
純化方法
Usual impurities are ethylbenzene, paraffins, traces of sulfur compounds and water. It is not practicable to separate the m-, and p-isomers of xylene by fractional distillation, although, with a sufficiently efficient still, o-xylene can be fractionally distilled from a mixture of isomers. Purify (and dry) by fractional distillation from LiAlH4, P2O5, CaH2 or sodium. This treatment can be preceded by shaking successively with conc H2SO4, water, aqueous 10% NaOH, water and mercury, and drying with CaCl2 for several days. Xylene can be purified by azeotropic distillation with 2-ethoxyethanol or 2-methoxyethanol, the distillate being washed with water to remove the alcohol, then dried and fractionally distilled. [Beilstein 5 H 360.]
キシレン 上流と下流の製品情報
原材料
準備製品
pesticide emulsifier BSL
polyurethane water-based emulsion finishes PU-II series
CHLOROMETHYL ISO-PROPYL ETHER
プロピソクロル
Pesticide emulsifier 0207
Fenvalerate+Malathion,E.C.(21%)
3-(ベンジルオキシ)フェノール
reversible temperature indicating coating (IV)
TOLUALDEHYDES
N,N'-ジフェニルベンジジン
Asphalt antifouling paint L40-32
pesticide emuslsifier 656L
p-キシリレンジアミン
2-クロロ-N-(1H-ピラゾール-1-イルメチル)-N-(2,6-ジメチルフェニル)アセトアミド
6-アミノ-2,4-ルチジン
4,4',7,7'-テトラメチル-5,5'-ジクロロ-Δ2,2'(3H,3'H)-ビ[ベンゾ[b]チオフェン]-3,3'-ジオン
1-アセチル-1,4-ジアゼパン
Amino resin varnish
pesticide emulsifier 6502
biodegrddable finishing agent for fabric
サンタロール
pesticide emulsifier 0206
SANTALYL ACETATE
CHLOROMETHYL BUTYL ETHER
Pyridaben E.C.
Malathion+Fenitrothion,E.C.
1,3,2-ジオキサチオラン2,2-ジオキシド
Quizalofop-ethyl E.C.
ジエチルベンゼン
ポリ(アクリル酸-CO-マレイン酸) 溶液
(2,6-Diisopropyl-4-Phenoxy)Phenylthiourea
Amino baking varnish
(3R,8aβ)-2,3,4,5,6,7,8,8a-オクタヒドロ-6-メトキシ-3β,6β,8,8-テトラメチル-1H-3aα,7α-メタノアズレン
1-メチルアミノ-1-メチルチオ-2-ニトロエチレン
キシリジン (混合物)
3-メチル-3-フェニルオキシラン-2-カルボン酸エチル
4-PROPYL-PYRIDIN-2-YLAMINE
3-イソキノリンカルボン酸メチル
pesticide emulsifier BCL
fire resistant silicone sealant LZ-850