N,N'-ジイソプロピルカルボジイミド 化学特性,用途語,生産方法
外観
無色~ほとんど無色透明液体
溶解性
水に可溶 (分解)。エタノール, エーテルに易溶。エタノール及びアセトンに極めて溶けやすく、水にほとんど溶けない。
用途
脱水縮合剤(15710の化学商品 (2010))ペプチド化学におけるカップリング試薬.(NTP DB(ACCESS ON NOV. 2010))
用途
ペプチド合成における縮合試薬。
使用上の注意
アルゴン封入
説明
Diisopropylcarbodiimide (DIC) is a clear liquid that can be easily dispensed by volume. It slowly reacts with moisture from the air, so for long term storage the bottle should be flushed with dry air or inert gas and sealed tightly. It is used in peptide chemistry as a coupling reagent. It is very toxic and caused contact dermatitis in a laboratory worker.
化学的特性
N,N'-Diisopropylcarbodiimide is colorless to pale yellow liquid. Insoluble in water, soluble in benzene, ethanol, ether.
使用
N,N'-Diisopropylcarbodiimide is used as a reagent in synthetic organic chemistry. It serves as a chemical intermediate and as a stabilizer for Sarin (chemical weapon). It is also used in the synthesis of peptide and nucleic acid. Further, it is used as an antineoplastic and involved in the treatment of malignant melanoma and sarcomas. It is mainly used in amikacin, glutathione dehydrants, as well as in synthesis of acid anhydride, aldehyde, ketone, isocyanate; when it is used as dehydrating condensing agent, it reacts to dicyclohexylurea through short-time reaction under normal temperature.
定義
ChEBI: A carbodiimide compound having an isopropyl substituent on both nitrogen atoms.
一般的な説明
N,N′-Diisopropylcarbodiimide (DIC) is a carbodiimide used as a coupling reagent in the synthesis of amides, peptides, ureas, heterocycles, and unsymmetrical carbodiimides. It is also used in the polymerization reactions as an activator.
接触アレルゲン
It is used in peptide chemistry as a coupling reagent. It
is very toxic and causes contact dermatitis in labora-
tory workers.
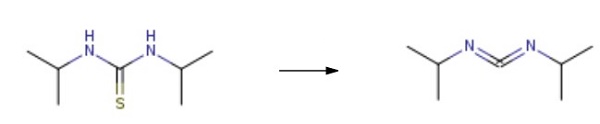
合成
Add N, N'diisopropylthiourea to the autoclave and use xylene as the solvent. The mass ratio of N, N'diisopropylthiourea to xylene solvent is 2:1. Add Sb2O4 catalyst (the amount of the catalyst is 1% of the total material), and oxygen is introduced. The temperature is increased to 115°C under a pressure of 8MPa for 4h, then lowered. Suction filtration at 15°C, decolorization, and vacuum distillation were used to obtain N, N'-Diisopropylcarbodiimide. The yield is 94.55%, and the purity is 99.55%.
N,N'-ジイソプロピルカルボジイミド 上流と下流の製品情報
原材料
準備製品
4-(ピペリジン-1-カルボニル)フェニルボロン酸
4-(2-CYANOETHYLAMINOCARBONYL)PHENYLBORONIC ACID
3-(2-CYANOETHYLAMINOCARBONYL)PHENYLBORONIC ACID
4-(3-TERT-BUTYL-1,2,4-OXADIAZOL-5-YL)PHENYLBORONIC ACID
3-TERT-BUTYL-5-(4-BROMOPHENYL)-1,2,4-OXADIAZOLE
4-(3-TERT-BUTYL-1,2,4-OXADIAZOL-5-YL)BENZALDEHYDE
O-tert-ブチル-N,N'-ジイソプロピルイソ尿素
4,5-Imidazolidinedione, 2,2-dichloro-1,3-bis(1-methylethyl)-
BIS(N N'-DIISOPROPYLACETAMIDINATO)NICKE&