アセトフェノン 化学特性,用途語,生産方法
外観
無色~うすい黄色, 澄明の液体
定義
本品は、次の化学式で表される有機化合物である。
溶解性
水に難溶 (0.55wt%, 20℃)。各種有機溶剤に可溶。エタノールに極めて溶けやすく、水に溶けにくい。
解説
アセトフェノン,芳香族ケトンの一つ。メチルフェニルケトン、アセチルベンゼンともいう。天然にはラブダナム油やウミダヌキ香に含まれている。ベンゼンと塩化アセチルとのフリーデル‐クラフツ反応により合成する。天然物から分離するには、蒸留したのち結晶化させる。独特の甘い芳香をもつ無色の液体で、冷却すると固化する。融点20 ℃,沸点202 ℃(100 kPa).1.0329.1.5363.水にはほとんど溶けないが、エタノール(エチルアルコール)、エーテルなどの有機溶媒にはよく溶ける。LD50 900 mg/kg(ラット,経口).濃硫酸と接触させると橙黄(とうこう)色となる。アルカリとハロゲンを反応させるとハロホルムCHX3(Xはハロゲン)と安息香酸C6H5COOHを生成する(ハロホルム反応)。香料製造の原料に用いられる。以前はヒプノンの名で催眠剤に用いた。
用途
着香料、溶剤、有機合成原料。
用途
溶剤、香料、医薬品?ケトン樹脂?農薬?ゴム薬原料
用途
香料その他の薬品の製造原料。ベンゼンと塩化アセチルを無水塩化アルミニウムを触媒とし反応させると得られる。ナッツ,タバコ,飲料,アイスクリーム,キャンディー,その他多くの食品に香料として使われる.イチゴや茶などの天然物にも少量含まれている.
合成
ととのフリーデル‐クラフツ反応により合成します。
毒性
飲み込むと有害であり、軽度の皮膚刺激性や強い眼刺激性を有します。また、本化合物は可燃性液体でもあります。このような理由から、消防法上では第4類第3石油類の非水溶性に、労働安全衛生法では有害物表示対象物に指定されています。
化粧品の成分用途
香料
説明
Acetophenone is the simplest aromatic ketone and is a clear liquid/crystal and very slightly
soluble in water with a sweet pungent taste and odour resembling oranges. It is used as
a polymerisation catalyst for the manufacture of olefins. Acetophenone is used in perfumery
as a fragrance ingredient in soaps, detergents, creams, lotions, and perfumes; as a
flavouring agent in foods, non-alcoholic beverages, and tobacco; as a specialty solvent for plastics and resins; as a catalyst for the polymerisation of olefins; and as a photosensitiser
in organic syntheses. Acetophenone is a raw material for the synthesis of some pharmaceuticals
and is also listed as an approved excipient by the U.S. FDA. Acetophenone occurs
naturally in many foods such as apple, apricot, banana, and beef. Acetophenone has been detected in ambient
air and drinking water; exposure of the general public may occur through the inhalation
of contaminated air or the consumption of contaminated water. It is highly flammable
and will get easily ignited by heat, sparks, or flames, and the vapours may form explosive
mixtures with air.
化学的特性
Acetophenone is a colorless, oily liquid with a sweet, floral odor.It is a naturally occurring component of a large number of foods and essential oils.
Acetophenone can be hydrogenated catalytically to 1-phenylethanol. It is obtained as a by-product in the Hock phenol synthesis and is purified from the high-boiling residue by distillation. The quantities obtained from this source satisfy the present demand.
Acetophenone is used for perfuming detergents and industrial products and is an intermediate in the synthesis of other fragrance materials.
天然物の起源
Reported found in cocoa, beef, raspberry, peas, and concord grape
使用
Acetophenone is a reagent used in the production of fragrances and resin polymers.
定義
ChEBI: Acetophenone is a methyl ketone that is acetone in which one of the methyl groups has been replaced by a phenyl group. It has a role as a photosensitizing agent, an animal metabolite and a xenobiotic.
調製方法
Most methyl phenyl ketone originates from the Hock process for the production of phenol from isopropylbenzene (→Phenol); it is isolated from the residue of this process. In addition, acetophenone can be obtained as a main product by selective decomposition of cumene hydroperoxide in the presence of copper catalysts at 100℃:
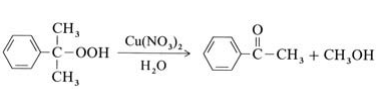
A second possibility is the oxidation of ethylbenzene with air or oxygen at 130℃ and 0.5 MPa. Catalysts used include cobalt salts or manganese salts of naphthenic or fatty acids. Conversion of ethylbenzene is limited to ca. 25 % to minimize the byproducts 1-phenylethanol and benzoic acid. A third method is the Friedel – Crafts acetylation of benzene with acetic anhydride, but this is not of industrial importance.
製造方法
From benzene and acetylchloride in the presence of aluminum chloride or by catalytic oxidation of ethyl benzene; also
prepared by fractional distillation and crystallization from the essential oil of Stirlingia latifolia.
一般的な説明
Acetophenone appears as a colorless liquid with a sweet pungent taste and odor resembling the odor of oranges. Freezes under cool conditions. Slightly soluble in water and denser than water. Hence sinks in water. Vapor heavier than air. A mild irritant to skin and eyes. Vapors can be narcotic in high concentrations. Used as a flavoring, solvent, and polymerization catalyst.
空気と水の反応
Slightly soluble in water.
反応プロフィール
Acetophenone reacts with many acids and bases liberating heat and flammable gases (e.g., H2). Reacts with many oxidizing agents. Reacts with reducing agents such as hydrides, alkali metals, and nitrides to produce flammable gas (H2) and heat. The amount of heat in these reactions may be sufficient to start a fire in the unreacted portion. Incompatible with isocyanates, aldehydes, cyanides, peroxides, and anhydrides.
健康ハザード
Acetophenone is an irritant, mutagen, and amildly toxic compound. In rabbits 0.77 mgproduced severe eye irritation, but the actionon skin was mild. In mice, subcutaneousadministration of this compound producedsleep; a dose of 330 mg/kg was lethal.
LD50 value, intraperitoneal (mice): 200mg/kg
No symptoms of severe toxicity, nor its carcinogenicityin humans, has been reported..
火災危険
Combustible liquid; flash point (closed cup)
82°C (180°F); vapor pressure 1 torr at
37°C (98.6°F); vapor density 4.1 (air = 1);
autoignition temperature 570°C (1058°F);
fire-extinguishing agent: dry chemical, foam,
or CO2; water may cause frothing, but it can
be used to flush and dilute the spill. Its reaction
with strong oxidizers may be violent.
使用用途
1. 独特の芳香と香料としての利用
アセトフェノンは、その独特の芳香を活かして、着香料や香料の合成原料として広く用いられています。その使用例としては、ナッツ,飲料,アイスクリーム,キャンディーなどの多くの食品やタバコなどが挙げられます。
2. ケト基の反応性の高さと工業製品、医薬品としての利用
アセトフェノンはカルボニル基を有するという構造上の特徴から工業製品、医薬品の有機合成における有用な基質となります。
安全性プロファイル
Poison by intraperitoneal and subcutaneous routesModerately toxic by ingestion. A skin and severe eye irritant. Mutation data reported. Narcotic in high concentration. A hypnotic. Flammable liquid. To fight fire, use foam, CO2, dry chemical. When heated to decomposition it emits acrid smoke and fumes. See also IGTONES
職業ばく露
Acetophenone is used as a solvent and in perfume manufacture to impact a pleasant jasmine or orange-blossom odor. It is used as a catalyst in olefin polymerization and as a flavorant in tobacco. It is also used in the synthesis of pharmaceuticals
発がん性
No carcinogenicity studies were
identified for acetophenone. The U.S. EPA has classified
acetophenone as a Category D, not classifiable as to human
carcinogenicity.
環境運命予測
It is unclear what mechanism is responsible for the central
nervous system depression observed following high doses of
acetophenone. In vitro evaluations have demonstrated that
acetophenone suppresses voltage-gated ion channels in olfactory
receptor cells and retinal neurons; however, it is unclear if
this is related to any of the observed toxicity in animal studies.
代謝
At one time, acetophenone was used as a hypnotic. Its conversion to benzoic acid and methylphenylcarbinol in dogs and rabbits was observed by a number of early workers. Small amounts are also excreted as mandelic acid. In the rabbit about half the dose is excreted as methylphenylcarbinyl glucuronide and about 20 % as hippuric acid. It is probable that the ketone is first asymmetrically reduced to the carbinol, which is the precursor of benzoic and mandelic acids.
合成方法
ベンゼンと水酢酸とのFriedel-Crafts反応により合成される。クメン法やHalcon法による副生成物として得られる。
輸送方法
UN1993 Flammable liquids, n.o.s., Hazard Class: 3; Labels: 3-Flammable liquid, Technical Name Required.
純化方法
Dry it by fractional distillation or by standing with anhydrous CaSO4 or CaCl2 for several days, followed by fractional distillation under reduced pressure (from P2O5, optional), and careful, slow and repeated partial crystallisations from the liquid at 0o excluding light and moisture. It can also be crystallised at low temperatures from isopentane. Distillation can be followed by purification using gas-liquid chromatography [Earls & Jones J Chem Soc, Faraday Trans 1 71 2186 1975.] [Beilstein 7 H 271, 7 IV 619.] § A commercial polystyrene supported version is available — scavenger resin (for diol substrates).
不和合性
May form explosive mixture with air. See flash point, above. Reacts violently with strong oxidizers, many acids, bases, amines, amides, and inorganic hydroxides; alkali metals; hydrides, and nitrides. Reacts with reducing agents; alkali metals; hydrides, nitrides. Contact with all preceding materials release heat and flammable gases, including hydrogen; the heat may be sufficient enough to result in fire. Incompatible with aldehydes, aliphatic amines, alkanolamines, cyanides, isocyanates, organic acids, peroxides; perchloric acid. May attack plastics, and some rubbers and coatings
廃棄物の処理
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal. Incineration, preferably with a flammable solvent
アセトフェノン 上流と下流の製品情報
原材料
準備製品
polyquinoxaline
3-Phenyl-1H-pyrazole-5-carboxamide ,97%
METHYL 2-AMINO-4-PHENYLTHIOPHENE-3-CARBOXYLATE
ホスホマイシンカルシウム
硝酸ミコナゾール
2-フェニルプロピオンアルデヒド
2-アミノ-4-フェニルチオフェン-3-カルボン酸エチル
FLUOXETINE HYDROCHLORIDE
(-)-ビス[(S)-1-フェニルエチル]アミン
(S,S)-(-)-ビス(α-メチルベンジル)アミン 塩酸塩
4-[3-(4-tert-ブチルフェニル)-2-メチルプロピル]-2,6-ジメチルモルホリン
2,2-ジクロロアセトフェノン
2,5-ジフェニルオキサゾール
DL-1-フェニルエチルアルコール
DL-1-フェニルエチルアミン
Alkofanone
(R,R)-(+)-ビス(α-メチルベンジル)アミン 塩酸塩
ナフチフィン
4,4,4-トリフルオロ-1-フェニル-1,3-ブタンジオン
3-アミノ-5-フェニルピラゾール
アセブトロール
3-フェニル-1H-ピラゾール-5-カルボヒドラジド
アゼラスチン
1-メチル-2-フェニルインドール
Enrofloxacin hydrochloride
3-ホルミル-1-メチル-2-フェニル-1H-インドール
METHYL ALPHA-BROMOPHENYLACETATE
1-フェニル-1,3-ブタンジオン
α-フェニルエタンアミン
ホスホマイシン
3-フェニル-1H-ピラゾール-5-カルボン酸
A-METHYL-3-PHENOXYBENZENEACETALDEHYDE
3,5-ジフェニル-1H-ピラゾール
エンロフロキサシン
3-フェニル-1H-ピラゾール-5-カルボン酸エチル
2-(2-イミノチアゾリジン-3-イル)-1-フェニルエタノン・臭化水素酸塩
3-メチル-3-フェニルオキシラン-2-カルボン酸エチル
5-PHENYL-3H-THIENO[2,3-D]PYRIMIDIN-4-ONE
エコナゾール
オキシフェドリン