아세트산 나트륨
|
|
아세트산 나트륨 속성
- 녹는점
- >300 °C (dec.)(lit.)
- 밀도
- 1.01 g/mL at 20 °C
- 굴절률
- 1.4640
- 인화점
- >250 °C
- 저장 조건
- Store at +5°C to +30°C.
- 용해도
- H2O: 3 M at 20 °C, 투명, 무색
- 산도 계수 (pKa)
- 4.756[at 20 ℃]
- 물리적 상태
- 가루
- 색상
- 하얀색
- Specific Gravity
- 1.45
- 냄새
- 가벼운 식초 냄새
- pH 범위
- 8.5 - 9.9 at 246 g/l at 25 °C
- 수소이온지수(pH)
- 7.87(1 mM solution);8.33(10 mM solution);8.75(100 mM solution);9.04(1000 mM solution)
- ?? ??
- 냄새 없는
- 수용성
- 500g/L(20℃)
- 감도
- Hygroscopic
- Hydrolytic Sensitivity
- 0: forms stable aqueous solutions
- 최대 파장(λmax)
- λ: 260 nm Amax: 0.03
λ: 280 nm Amax: 0.02
- Merck
- 14,8571
- BRN
- 3595639
- 끓는 점
- >400°C(decomposition)
- 안정성
- 안정적인. 강한 산화제, 할로겐과 호환되지 않습니다. 수분에 민감합니다.
- InChIKey
- VMHLLURERBWHNL-UHFFFAOYSA-M
- LogP
- -3.72
- CAS 데이터베이스
- 127-09-3(CAS DataBase Reference)
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 안전지침서 | 22-24/25 | ||
|---|---|---|---|
| WGK 독일 | 1 | ||
| RTECS 번호 | AJ4300010 | ||
| F 고인화성물질 | 3 | ||
| 자연 발화 온도 | 607 °C | ||
| TSCA | Yes | ||
| HS 번호 | 29152200 | ||
| 유해 물질 데이터 | 127-09-3(Hazardous Substances Data) | ||
| 독성 | LD50 orally in Rabbit: 3530 mg/kg LD50 dermal Rabbit > 10000 mg/kg | ||
| 기존화학 물질 | KE-00061 |
아세트산 나트륨 C화학적 특성, 용도, 생산
물성
백색의 결정성분말 또는 덩어리이고 냄새가 없거나 신냄새가 있다(무수화물) 무색투명의 결정 또는 백색의 결정성분말이다(3수화물), pH 7.5~9.5, 녹는점 324℃(무수화물) 79℃(3수화물), 끓는점 881.4℃(무수화물) 120~123℃(3수화물), 인화점 >250℃(무수화물) 300℃(3수화물), 용해도 465g/ℓ(무수화물) 49781㎎/ℓ(3수화물), 비중 1.528 (무수화물) 1.4442 (3수화물), 분자량 82.04(무수화물) 137.11(3수화물)순도시험
(1) 용상 : 이 품목 1g을 취하여 물 20mL에 녹일 때, 그 액은 무색으로서 탁도는 징명하여야 한다.
(2) 유리산 및 유리알칼리 : 결정물일 때는 2g, 무수물일 때는 1.2g을 취하여 새로 끓여서 식힌 물 20mL에 녹여 페놀프탈레인시액 2방울을 가한 다음 10℃로 유지하여 다음의 시험을 행한다.
① 액이 무색이면, 0.1N 수산화나트륨용액 0.1mL를 가할 때, 홍색을 나타내어야 한다.
② 액이 홍색이면, 그 색은 0.1N 염산 0.1mL를 가할 때, 없어져야 한다.
(3) 비소 : 이 품목을 비소시험법에 따라 시험할 때, 그 양은 4.0ppm 이하이어야 한다.
(4) 납 : 이 품목 5.0g을 취하여 원자흡광광도법 또는 유도결합플라즈마발광광도법에 따라 시험할 때, 그 양은 2.0ppm 이하이어야 한다.
(5) 수은 : 이 품목을 수은시험법에 따라 시험할 때, 그 양은 1.0ppm 이하이어야 한다.
확인시험
(1) 이 품목을 서서히 가열하면 용해되고 다음에 분해하여 아세톤의 냄새가 난다. 또 잔류물의 수용액은 알칼리성이다.
(2) 이 품목은 확인시험법 중 나트륨염 및 초산염의 반응을 나타낸다.
정량법
이 품목을 건조한 다음 약 0.2g을 정밀히 달아 초산 40mL를 가하여 녹이고 0.1N 과염소산용액으로 적정한다. 종말점의 확인은 보통 전위차를 이용한다(지시약 : 크리스탈바이올렛․빙초산시액 1mL). 다만, 종말점은 액의 자색이 청색을 지나 녹색으로 변할 때로 한다. 따로 공시험을 행하여 보정한다.
0.1N 과염소산용액 1mL = 8.203mg C2H3NaO2
정의
이 품목에는 결정물(3수염) 및 무수물이 있고, 각각을 초산나트륨(결정) 및 초산나트륨(무수)이라 칭한다.
개요
Sodium acetate (CH3COONa) is the sodium salt of acetic acid. It appears as a colorless deliquescent salt with a wide range of applications. In industry, it can be used in textile industry to neutralize sulfuric acid waste streams and as a photoresist upon using aniline dyes. In concrete industry, it can be used as a concrete sealant to mitigate the water damage. In food, it can be used as a seasoning. It can also be used as a buffer solution in lab. In addition, it is also used in heating pads, hand warmers and hot ice. For laboratory use, it can be produced by the reaction between acetate with the sodium carbonate, sodium bicarbonate and sodium hydroxide. In industry, it is prepared from the glacial acetic acid and sodium hydroxide.화학적 성질
Sodium acetate, CH3COONa, also abbreviated NaOAc , also sodium ethanoate, is the sodium salt of acetic acid, was made by the reaction of acetic acid with sodium carbonate. It is soluble in water but less so in alcohol. This colourless salt has a wide range of uses. Sodium acetate was used as a pH modifier for toning baths.물리적 성질
Anhydrous salt is a colorless crystalline solid; density 1.528 g/cm3; melts at 324°C; very soluble in water; moderately soluble in ethanol. The colorless crystalline trihydrate has a density 1.45 g/cm3; decomposes at 58°C; is very soluble in water; pH of 0.1M aqueous solution is 8.9; moderately soluble in ethanol, 5.3 g/100mL.출처
Acetic acid or acetates are present in most plant and animal tissues in small, but detectable amounts용도
Used as buffers.Acidity regulation (buffering)
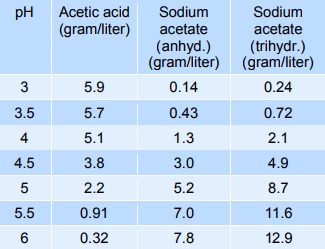
Sodium acetate mixed with acetic acid forms a pH buffer, which can be used to stabilise the pH of foods in the pH-range from 3 to 6. The table below gives indicative values of the composition needed to give a certain pH. The mixtures below can be diluted at least 10 times with minimum effect on pH, however, the stability decreases.
제조 방법
Sodium acetate is prepared by reacting sodium hydroxide or sodium carbonate with acetic acid in aqueous solution. The solution is evaporated to obtain hydrated crystals of sodium acetate.NaOH + CH3COOH → CH3COONa + H2O
Na2CO3 + CH3COOH → 2CH3COONa + CO2 + H2O
정의
ChEBI: Sodium acetate is an organic sodium salt. It contains an acetate.Synthesis
For laboratory use, sodium acetate is very inexpensive, and is usually purchased instead of being synthesized. It is sometimes produced in a laboratory experiment by the reaction of acetic acid (ethanoic acid) with sodium carbonate, sodium bicarbonate, or sodium hydroxide. These reactions produce aqueous sodium acetate and water. Carbon dioxide is produced in the reaction with sodium carbonate and bicarbonate, and it leaves the reaction vessel as a gas (unless the reaction vessel is pressurized). This is the well-known "volcano" reaction between baking soda (sodium bicarbonate) and vinegar.CH3COOH + NaHCO3 → CH3COONa + H2O + CO2
Industrially, sodium acetate is prepared from glacial acetic acid and sodium hydroxide.
CH3COOH + NaOH → CH3COONa + H2O.
화학 반응
Sodium acetate can be used to form an ester with an alkyl halide such as bromo ethane:CH3COONa + Br CH2CH3→ CH3COOCH2CH3+ NaBr
Caesium salts catalyze this reaction.
일반 설명
Sodium Acetate is reported to inhibit the growth of Listeria monocytogenes.반응 프로필
When sodium acetate reacts with strong acids, irritating, noxious vapors of acetic acid are usually produced. Sodium acetate is sufficiently basic to catalyze the violent polymerization of diketene, perhaps as well as other reactive dimers that are susceptible to polymerization in the presence of a mild base.생물학적 활성
Commonly used laboratory reagentSafety Profile
Poison by intravenous route. Moderately toxic by ingestion. A skin and eye irritant. Migrates to food from packagmg materials. Violent reaction with F2, m03, diketene. When heated to decomposition it emits toxic fumes of Na2O.Purification Methods
Crystallise it from acetic acid and keep it under vacuum for 10hours at 120o. Alternatively, it is crystallised from aqueous EtOH, as the trihydrate. This material can be converted to anhydrous salt by heating slowly in a porcelain, nickel or iron dish, so that the salt liquefies. Steam is evolved and the mass again solidifies. Heating is now increased so that the salt melts again. (NB: if it is heated too strongly, the salt can char; avoid this.) After several minutes, the salt is allowed to solidify and is cooled to a convenient temperature (in a desiccator) before being powdered and bottled. The water content should now be less than 0.02%. [Beilstein 2 II 113, 2 III 184, 2 IV 109.]아세트산 나트륨 준비 용품 및 원자재
원자재
준비 용품
아세트산 나트륨 공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Jiangsu Kolod Food Ingredients Co.,Ltd. | +86-518-85110578 +8618805133257 |
sales3257@jskolod.com | China | 132 | 60 |
| Shanghai UCHEM Inc. | +862156762820 +86-13564624040 |
sales@myuchem.com | China | 7433 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 |
info@fdachem.com | China | 20288 | 58 |
| Hebei Saisier Technology Co., LTD | +86-18400010335 +86-18034520335 |
admin@hbsaisier.cn | China | 1015 | 58 |
| Aladdin Scientific | +1-+1(833)-552-7181 |
sales@aladdinsci.com | United States | 57505 | 58 |
| HebeiShuoshengImportandExportco.,Ltd | +86-18532138899 +86-18532138899 |
L18532138899@163.com | China | 939 | 58 |
| SHANGHAI KEAN TECHNOLOGY CO., LTD. | +8613817748580 |
cooperation@kean-chem.com | China | 40066 | 58 |
| Wuhan Boyuan Import & Export Co., LTD | +8615175982296 |
Mike@whby-chem.com | China | 33 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-29-81148696 +86-15536356810 |
1022@dideu.com | China | 3882 | 58 |
| Nanjing Deda New Material Technology Co., Ltd | +8613223293093 |
bella@njdeda.com | China | 80 | 58 |