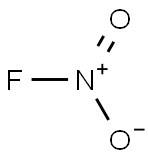
nitryl fluoride C화학적 특성, 용도, 생산
화학적 성질
Colorless gas.Strong oxidizing agent; hydrolyzes to
form nitric and hydrogen fluoride acids.
물리적 성질
Colorless gas; pungent odor; density 2.90 g/L; heavier than air, density in air 2.24 (air=1); liquefies to a colorless liquid at -63.5°C; solidifies at -139°C; decomposes in water; also decomposes in alcohol, ether and chloroform.
용도
Oxidizer in rocket propellants; fluorinating agent.
위험도
Ignites on contact with selenium, iodine,
phosphorus, arsenic, antimony, boron, silicon,
molybdenum. Corrosive to tissue.
Safety Profile
Poison by inhalation. A
severe irritant to skin, eyes, and mucous
membranes. A powerful oxidlzing agent.
This gas is intensely reactive. Explosive
reaction with hydrogen at 200-300°C.
Ignites on contact with antimony, arsenic,
boron, iodme, phosphorus, selenium. Ignites
when warmed with bismuth, carbon,
chromium, lead, sulfur. Incandescent
reaction with aluminum, cadmium, cobalt,
iron, molybdenum, nickel, potassium,
sodium, thorium, titanium, tungsten, uran uranium,
vanadlum, zinc, zirconium, lithium (at
200-300°C), manganese (at 200-300°C).
Incompatible with metals, nonmetals. When
heated to decomposition it emits toxic
fumes of Fand NOx. See also
FLUORIDES.
nitryl fluoride 준비 용품 및 원자재
원자재
준비 용품