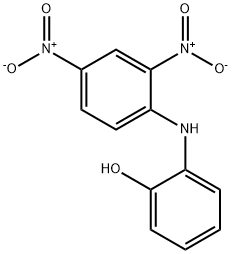
2-(2,4-디니트로아닐리노)페놀 C화학적 특성, 용도, 생산
화학적 성질
orange crystalline powder
일반 설명
Orange crystals.
공기와 물의 반응
Insoluble in water.
반응 프로필
2-(2,4-dinitroanilino)phenol is an organonitrate/phenol. Organonitrate compounds range from slight to strong oxidizing agents. If mixed with reducing agents, including hydrides, sulfides and nitrides, they may begin a vigorous reaction that culminates in a detonation. The aromatic nitro compounds may explode in the presence of a base such as sodium hydroxide or potassium hydroxide even in the presence of water or organic solvents. The explosive tendencies of aromatic nitro compounds are increased by the presence of multiple nitro groups. Phenols do not behave as organic alcohols, as one might guess from the presence of a hydroxyl (-OH) group in their structure. Instead, they react as weak organic acids. Phenols and cresols are much weaker as acids than common carboxylic acids (phenol has Ka = 1.3 x 10^[-10]). These materials are incompatible with strong reducing substances such as hydrides, nitrides, alkali metals, and sulfides. Flammable gas (H2) is often generated, and the heat of the reaction may ignite the gas. Heat is also generated by the acid-base reaction between phenols and bases.
화재위험
Flash point data are not available for 2-(2,4-dinitroanilino)phenol, but 2-(2,4-dinitroanilino)phenol is probably combustible.
2-(2,4-디니트로아닐리노)페놀 준비 용품 및 원자재
원자재
준비 용품