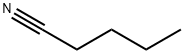
발레로니트릴 C화학적 특성, 용도, 생산
화학적 성질
Clear liquid
용도
Valeronitrile is used as building block in organic synthesis. Product Data Sheet
생산 방법
Valeronitrile can be synthesized by dehydration of valeronamide. The nitrile is
also found in nature and is a constituent of coal gasification and oil shale
processing waste water, sewage wastewater
and tobacco smoke.
일반 설명
Clear colorless to yellow liquid.
공기와 물의 반응
Slightly soluble in water.
반응 프로필
Nitriles, such as Valeronitrile, may polymerize in the presence of metals and some metal compounds. They are incompatible with acids; mixing nitriles with strong oxidizing acids can lead to extremely violent reactions. Nitriles are generally incompatible with other oxidizing agents such as peroxides and epoxides. The combination of bases and nitriles can produce hydrogen cyanide. Nitriles are hydrolyzed in both aqueous acid and base to give carboxylic acids (or salts of carboxylic acids). These reactions generate heat. Peroxides convert nitriles to amides. Nitriles can react vigorously with reducing agents. Acetonitrile and propionitrile are soluble in water, but nitriles higher than propionitrile have low aqueous solubility. They are also insoluble in aqueous acids. Valeronitrile is incompatible with strong acids, strong bases, strong oxidizing agents and strong reducing agents. .
건강위험
Valeronitrile is an irritant and may be harmful by inhalation, ingestion or skin
absorption .
화재위험
Valeronitrile is combustible.
공업 용도
Valeronitrile is used as an industrial solvent and as a chemical intermediate.
Toxicology
Pentanenitrile is toxic to animals, and produces its action by the liberation of cyanide by cytochrome P450. The cyanide is detoxified and excreted in urine as thiocyanate.
신진 대사
As with other aliphatic nitriles, valeronitrile is metabolized in vivo resulting in the
liberation of cyanide ion which is responsible for much of the observed toxicity of
this compound .
Biotransformation of valeronitrile presumably proceeds in a manner similar to that
of other aliphatic nitriles with an initial cytochrome P-450 catalyzed oxidation of
the nitrile to the cyanohydrin followed by release of the cyanide group from the
activated molecule. Cyanide formation was significantly reduced when
valeronitrile was incubated with mouse hepatic microsomes in the presence of
SKF-525A or carbon monoxide or when microsomes from mice pretreated with
chloroform were used . Ethanol pretreatment of mice markedly
increases the in vivo and in vitro microsomal oxidation of valeronitrile presumably as a result of increased levels of an ethanol inducible
cytochrome P-450 . As with other nitriles, the cyanide released
upon biotransformation of valeronitrile is readily converted to thiocyanate in vivo
and the latter ion was the major urinary excretion product observed with valero-nitrile in rats . From 18 to 31% of a daily 175 mg/kg dose of
valeronitrile was eliminated in the urine as thiocyanate during a 24 h period. In
another study , 43.2 and 27.5%, respectively, of an oral or i.p.
dose of 0.75 mmol/kg valeronitrile was excreted as thiocyanate in the urine of
male Sprague-Dawley rats over a 24 h period.
Purification Methods
Wash the nitrile with half its volume of conc HCl (twice), then with saturated aqueous NaHCO3, dry it with MgSO4 and fractionally distil it from P2O5. [Beilstein 2 H 301, 2 I 131, 2 II 267, 2 III 675, 2 IV 875.]
Structure and conformation
The valeronitrile molecule is flexible and can adopt a number of different conformers, so that it will naturally be a mixture. These conformers are called anti-anti (30%), anti-gauche (46%), gauche-anti, gauche-gauche-cis, and gauche-gauche-trans.
발레로니트릴 준비 용품 및 원자재
원자재
준비 용품