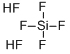플루오로규산
|
|
플루오로규산 속성
- 끓는 점
- 108-109°C
- 밀도
- 1.22 g/mL at 20 °C (lit.) 1.31 g/mL at 25 °C
- 증기압
- 23hPa at 19.85℃
- 굴절률
- 1.3500
- 인화점
- 108-109°C
- 저장 조건
- −20°C
- 용해도
- H2O: 1 mg/mL, 투명, 무색
- 산도 계수 (pKa)
- 1.83[at 20 ℃]
- 물리적 상태
- 액체
- 색상
- 무색의
- Specific Gravity
- 1.38 (40%)
- 수용성
- 물과 섞일 수 있습니다.
- Hydrolytic Sensitivity
- 0: forms stable aqueous solutions
- Merck
- 14,4182
- 노출 한도
- ACGIH: TWA 2.5 mg/m3
NIOSH: IDLH 250 mg/m3; TWA 2.5 mg/m3
- 안정성
- 수용액에서는 안정하다.
- InChIKey
- AUJBMDCSBIPDEH-UHFFFAOYSA-N
- CAS 데이터베이스
- 16961-83-4(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | C | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 34-35-20/21/22 | ||
| 안전지침서 | 26-36/37/39-45-27 | ||
| 유엔번호(UN No.) | UN 1778 8/PG 2 | ||
| WGK 독일 | 3 | ||
| RTECS 번호 | VV8225000 | ||
| F 고인화성물질 | 8-10 | ||
| 위험 참고 사항 | Corrosive | ||
| TSCA | Yes | ||
| 위험 등급 | 8 | ||
| 포장분류 | II | ||
| HS 번호 | 28111990 | ||
| 유해 물질 데이터 | 16961-83-4(Hazardous Substances Data) | ||
| 독성 | LD50 oral in rat: 430mg/kg | ||
| 기존화학 물질 | KE-18550 | ||
| 유해화학물질 필터링 | 97-1-376 | ||
| 함량 및 규제정보 | 물질구분: 유독물질; 혼합물(제품)함량정보: 플루오로규산과 그 염류 및 그 중 하나를 1% 이상 함유한 혼합물 |
플루오로규산 C화학적 특성, 용도, 생산
개요
Hexafluorosilicic acid is a kind of inorganic acid. It is majorly used for the fluoridation of water in United State to minimize the incidence of dental caries and dental fluorosis. For chemical synthesis, it is majorly used for the manufacturing of aluminum fluoride and cryolite as well as many kinds of hexafluorosilicate salts. It can also be used for the production of silicon and silicon dioxide. It can also be used as an electrolyte in the Betts electrolytic process for refining lead. It is also a specialized reagent in organic synthesis for cleaving Si–O bonds of silyl ethers.화학적 성질
Fluosilicic acid,H2SiF6, also known as hydrofluorosilicic acid,is a colorless liquid that is soluble in water. It is highly corrosive and toxic,attacking glass and stoneware. Fluosilicic acid is used in water fluoridation, electroplating, and in manufacturing enamels and cement.물리적 성질
d 1.220 g cm?3 for a 25% aq solution.용도
Hexafluorosilicic acid is commonly used as a source of fluoride. It is converted to a variety of useful hexafluorosilicate salts. It is also used as an electrolyte in the Betts electrolytic process for refining lead. It is an important organic reagent for cleaving Si-O bonds of silyl ethers. Further, it is used as wood a preservation agent and also used in surface modification of calcium carbonate.일반 설명
A colorless fuming liquid with a penetrating pungent odor. Corrosive to metals and tissue. Both the fumes and very short contact with the liquid can cause severe and painful burns. Used in water fluoridation, in hardening cement and ceramics, as a wood preservative.공기와 물의 반응
Fumes in air. Soluble in water with release of heat and corrosive fumes.반응 프로필
Hexafluorosilicic acid can react with strong acids (such as sulfuric acid) to release fumes of toxic hydrogen fluoride. Attacks glass and materials containing silica. Reacts exothermically with chemical bases (examples: amines, amides, inorganic hydroxides). Reacts with active metals, including iron and aluminum to dissolve the metal and liberate hydrogen and/or toxic gases. Can initiate polymerization in certain alkenes. Reacts with cyanide salts and compounds to release gaseous hydrogen cyanide. Flammable and/or toxic gases are also often generated by reactions with dithiocarbamates, isocyanates, mercaptans, nitrides, nitriles, sulfides, and weak or strong reducing agents. Additional gas-generating reactions may occur with sulfites, nitrites, thiosulfates (to give H2S and SO3), dithionites (SO2), and carbonates. Can catalyze (increase the rate of) chemical reactions. Decomposes when heated to the boiling point to produce very toxic and corrosive hydrogen fluoride gas.위험도
Extremely corrosive by skin contact and inhalation.건강위험
Inhalation of vapor produces severe corrosive effect on mucous membrane. Ingestion causes severe burns of mouth and stomach. Contact with liquid or vapor causes severe burns of eyes and skin.화재위험
Special Hazards of Combustion Products: Irritating fumes of hydrogen fluoride may form in fire.공업 용도
Hydrofluorosilicic acid (H2SiF6) is a colorless to light brown liquid. It is also manufactured from calcium fluoride or other fluoride-containing products. Hydrofluorosilic acid is a strong depressant for many silicates during flotation of a number of oxidic minerals. It is used for gangue depression during flotation of tin, columbite and tantalite.Safety Profile
Poison by subcutaneous route. A corrosive irritant to sktn, eyes, and mucous membranes. Will react with water or steam to produce toxic and corrosive fumes. When heated to decomposition it emits toxic fumes of F-. See also FLUORIDES.잠재적 노출
A solution of fluorosilicic acid is used for sterilization in the brewing and bottling industry, elec trolytic refining of lead; electroplating, hardening cement; removing mold, and others.운송 방법
UN1778 Fluorosilicic acid, Hazard class: 8; Labels: 8-Corrosive material.비 호환성
The aqueous solution is a strong acid. Reacts with water or steam to produce toxic and corrosive fumes of hydrogen fluoride. Incompatible, and may react violently with: bases, aliphatic amines; alkanolamines, alkylene oxides; aromatic amines; amides, ammonia, ammonium hydroxide; calcium oxide; epichlorohydrin, iso cyanates, oleum, organic anhydrides; sulfuric acid; strong oxidizers; vinyl acetate; water. Attacks glass, concrete, and ceramics. The anhydrous form dissociates almost instantly into silicon tetrafluoride and hydrogen fluoride.폐기물 처리
Add slowly to a large amount of soda ash in solution. Discharge to sewer with large volumes of water참고 문헌
Robinson, Tim. "Innovative Processes in Electrometallurgy." Innovative Process Development in Metallurgical Industry. Springer International Publishing, 2016. 385- 392.Sarawade, Pradip B., et al. "Recovery of high surface area mesoporous silica from waste hexafluorosilicic acid (H 2 SiF 6) of fertilizer industry." Journal of hazardous materials 173.1 (2010): 576-580.
Kauffman, Joel M. "Water fluoridation: a review of recent research and actions." Journal of American Physicians and Surgeons 10.2 (2005): 38.
Krot, V. V., et al. "ChemInform Abstract: Preparation of Amorphous Silicon Dioxide from Hexafluorosilicic Acid." Cheminform 23.48(1992):no-no.
Zorya, L., and V. Krot. "Method of high-purity silica production from hexafluorosilicic acid." Reaction Kinetics & Catalysis Letters 50.1-2(1993):349-354.
플루오로규산 준비 용품 및 원자재
원자재
준비 용품
플루오로규산 공급 업체
글로벌( 368)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Hebei Chuanghai Biotechnology Co., Ltd | +8617732866630 |
abby@chuanghaibio.com | China | 8773 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 |
sales1@chuanghaibio.com | China | 5251 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21622 | 55 |
| career henan chemical co | +86-0371-86658258 +8613203830695 |
sales@coreychem.com | China | 29855 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 |
linda@hubeijusheng.com | CHINA | 28172 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 |
linda@hubeijusheng.com | CHINA | 22963 | 58 |
| Hebei Mujin Biotechnology Co.,Ltd | +86-13288715578 |
sales@hbmojin.com | China | 12748 | 58 |
| SIMAGCHEM CORP | +86-13806087780 |
sale@simagchem.com | China | 17346 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418671 +8618949823763 |
sales@tnjchem.com | China | 34563 | 58 |
| Wuhan Monad Medicine Tech Co.,LTD | 02768782018 18771942761 |
sales01@whmonad.com | CHINA | 979 | 58 |