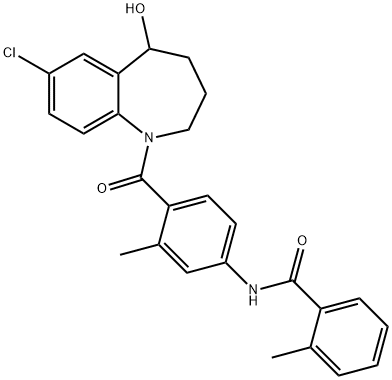
Tolvaptan C화학적 특성, 용도, 생산
개요
The potent antidiuretic hormone AVP orchestrates the regulation of
free water absorption, body fluid osmolality, cell contraction, blood
volume, and blood pressure through stimulation of three G-proteincoupled receptor subtypes:V
1-vascular types a and b, V
2-renal, and
V
3-pituitary. Increased AVP secretion is the trademark of several pathophysiological disorders, including heart failure, impaired renal function, liver cirrhosis, and SIADH. As a consequence, these patients
experience excess water retention or inadequate free-water excretion,
which results in the dilution of sodium concentrations, frequently
manifesting as clinical hyponatremia (serum sodium concentration
<135 mmol/L). This electrolyte imbalance increases mortality rates by
60-fold. Selective antagonism of the AVP V
2receptor promotes water excretion without perturbing electrolyte balance making it an appealing
target for preventing disease progression. Following the introduction of
the dual AVP V
1a/V
2 receptor antagonist conivaptan, tolvaptan has
recently been launched as a nonpeptide, selective V
2 receptor antagonist with potent aquaretic attributes for the treatment of hypervolemic
and euvolemic hyponatremia (serum sodium concentration of
<125 mmol/L or less distinct hyponatremia that is symptomatic and
has resisted correction with fluid restriction).
As a more potent and
selective V
2 receptor antagonist, tolvaptan is a follow-up to mozavaptan, which possesses weak V
1 receptor antagonism and was approved
for the treatment of SIADH in Japan.
.
화학적 성질
White Solid
용도
Labelled Tolvaptan
s, and the syndrome of inappropriate antidiuretic hormone (SIADH).
Clinical Use
Tolvaptan, also known as OPC-41061, is a selective, competitive
arginine vasopressin receptor 2 antagonist used to treat hyponatremia
(low blood sodium levels) associated with congestive heart
failure, cirrhosis, and the syndrome of inappropriate antidiuretic
hormone (SIADH). Otsuka Pharmaceutical licensed tolvaptan
under the trade name Samsca after the FDA approved the drug in
May 2009. Tolvaptan has also shown efficacy against polycystic
kidney disease. In a 2004 trial, tolvaptan administered with traditional
diuretics was noted to increase excretion of excess fluids and
improve blood sodium levels in patients with heart failure without
producing side effects such as hypotension (low blood pressure) or
hypokalemia (decreased blood levels of potassium). The drug also
exhibited no adverse effect on kidney function.
부작용
The most common adverse events were thirst, dry mouth, asthenia, constipation, pollakiuria or polyuria, and hyperglycemia. The recommended starting dose is 15 mg daily with a daily 15-mg adjustment to a maximum of 60 mg daily to raise serum sodium concentration. Initiation should be in a hospital setting where serum sodium and volume status may be monitored since too rapid correction of hyponatremia (>12 mEq/L/24 h) can cause osmotic demyelination resulting in dysarthria, mutism, dysphagia, lethargy, spastic quadriparesis, seizures, coma, and death. In addition to avoiding concomitant use of strong CYP3A4 inhibitors, tolvaptan is contraindicated in settings of urgent need to raise serum sodium acutely, in patients with an inability to sense or appropriately respond to thirst, in hypovolemic hyponatremia conditions, and in anuric patients.
Tolvaptan 준비 용품 및 원자재
원자재
준비 용품