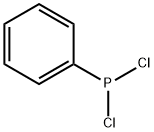
벤젠포스포너스디클로라이드 C화학적 특성, 용도, 생산
화학적 성질
Colorless to light yellow liqui
용도
Dichlorophenylphosphine and its derivatives are used as an intermediate to make plasticizers, antioxidants, stabilizers, catalysts and high pressure lubricant additives and in lacquer formulatings.
일반 설명
A colorless, fuming liquid. Density 1.32 g / cm3. Flash point 102°C. Corrosive to metals and tissue.
공기와 물의 반응
Reacts with water, including moisture in air or soil, to liberate heat and hydrochloric acid [Merck, 11th ed. 1989].
반응 프로필
Dichlorophenylphosphine is incompatible with water, with bases (including amines), with strong oxidizing agents, and with alcohols. May react vigorously or explosively if mixed with diisopropyl ether or other ethers in the presence of trace amounts of metal salts [J. Haz. Mat., 1981, 4, 291].
위험도
Highly corrosive to skin, tissue. Flammable.
건강위험
Inhalation causes irritation of nose and throat; pulmonary edema may develop following severe exposures. Contact with skin or eyes causes severe burns. Ingestion causes severe burns of mouth and stomach.
Safety Profile
A poison irritant to
skin, eyes, and mucous membranes and
poison by ingestion and inhalation.
When heated to decomposition it emits
very toxic fumes of Cland POx. See
also PHOSPHINE.
Purification Methods
Distil it in a vacuum byfractionating through a 20cm column packed with glass helices (better use a spinning band column) [Buchner & Lockhart J Am Chem Soc 73 755 1951, NMR: Müller et al. J Am Chem Soc 78 3557 1956, IR: Daasch & Smith Anal Chem 23 853 1951]. It forms a yellow Ni complex: Ni(C6H5Cl2P)4 (m 91-92o, from H2O) [Quin J Am Chem Soc 79 3681 1957 ] and a yellow complex with molybdenum carbonyl: Mo(CO)3.(C6H5Cl2P)3 (m 106-110o dec) [Abel et al. J Chem Soc 2323 1959]. [Beilstein 16 IV 972.]
벤젠포스포너스디클로라이드 준비 용품 및 원자재
원자재
준비 용품