아세나프텐 C화학적 특성, 용도, 생산
개요
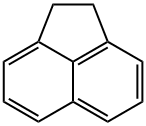
Acenaphthene is a tricyclic aromatic hydrocarbon and crystalline solid at ambient
temperature. Acenaphthene does not dissolve in water but is soluble in many organic
solvents. Acenaphthene is a component of crude oil and a product of combustion.
Acenaphthene occurs in coal tar produced during the high-temperature carbonisation
or coking of coal. It is used as a dye intermediate in the manufacture of some plastics
and as an insecticide and fungicide. Acenaphthene is a component of crude oil
and a product of combustion which may be produced and released to the environment
during natural fires. Emissions from petroleum refining, coal tar distillation, coal
combustion, and diesel-fuelled engines are major contributors of acenaphthene to the
environment. Acenaphthene is an environmental pollutant and has been detected in
cigarette smoke, automobile exhausts, and urban air; in effluents from petrochemical,
pesticide, and wood preservative industries; and in soils, groundwater, and surface
waters at hazardous waste sites. This compound is one among a number of polycyclic
aromatic hydrocarbons (PAHs) on U.S. EPA’s (Environmental Protection Agency)
priority pollutant list.
화학적 성질
Acenaphthene is a tricyclic aromatic hydrocarbon, crystalline solid at ambient tempera-
ture. Acenaphthene does not dissolve in water, but is soluble in many organic solvents.
Acenaphthene occurs in coal tar produced during high temperature carbonization or cok-
ing of coal. It is used as a dye intermediate in the manufacture of some plastics and as
an insecticide and fungicide. Acenaphthene is a component of crude oil and a product
of combustion that may be produced and released into the environment during natural
fi
res. Emissions from petroleum refi
ning, coal tar distillation, coal combustion, and diesel-
fueled engines are major contributors of acenaphthene to the environment. Acenaphthene
is an environmental pollutant and has been detected in cigarette smoke, automobile
exhausts, and urban air; in effl
uents from petrochemical, pesticide, and wood preservative
industries; and in soils, groundwater, and surface waters at hazardous waste sites. This
compound is one of a number of polycyclic aromatic hydrocarbons on the US EPA’s prior-
ity pollutant list.
물리적 성질
White crystalline solid or orthorhombic bipyramidal needles from alcohol. Coal tar-like odor. The
lowest odor threshold concentration in water that may result in rejection of contaminated water
ranged from 0.02 to 0.22 ppm (Lillard and Powers, 1975). In Wisconsin, the taste and odor
threshold concentration in water that is nontoxic to humans is 20 μg/L (ATSDR, 1995).
용도
Acenaphthene occurs in petroleum bottoms and is used as a
dye intermediate, insecticide, and fungicide and in
manufacturing plastics.
정의
acenaphthene: A colourless crystallinearomatic compound, C
12H
10;m.p. 95°C; b.p. 278°C. It is an intermediatein the production of somedyes.
일반 설명
White needles. Melting point 93.6°C. Soluble in hot alcohol. Denser than water and insoluble in water. Hence sinks in water. May irritate skin and mucous membranes. Emits acrid smoke and irritating fumes when heated to decomposition. Derived from coal tar and used to make dyes, pharmaceuticals, insecticides, fungicides, and plastics.
공기와 물의 반응
Insoluble in water.
반응 프로필
Acenaphthene is incompatible with strong oxidizing agents. Incompatible with ozone and chlorinating agents. Forms crystalline complexes with desoxycholic acid .
건강위험
Exposures to acenaphthene cause poisoning and include symptoms such as irritation
to the skin, eyes, mucous membranes, and upper respiratory tract. Studies on labora-
tory animals orally exposed to acenaphthene showed loss of body weight, peripheral
blood changes (unspecifi
ed), increased aminotransferase levels in blood serum, and
mild morphological damage to the liver and kidneys. In chronic exposures, acenaph-
thene is known to cause damage to the kidneys and liver. Acenaphthene is irritating to
the skin and mucous membranes of humans and animals. Oral exposure of rats to ace-
naphthene for 32 days produced peripheral blood changes, mild liver and kidney dam-
age, and pulmonary effects. However, detailed studies with acenaphthene in humans
are limited.
화재위험
Flash point data for Acenaphthene are not available. Acenaphthene is probably combustible.
Safety Profile
Moderately toxic by intraperitonealroute. Mutation data reported.Incompatible with strongoxidizing agents, ozone, chlorinating agents. When heatedto decomposition it emits acrid smoke and irritating vapors.
잠재적 노출
Acenaphthene occurs naturally in coal tar and in coal tar produced during the high-temperature carbonization or coking of coal; coal tar distilling; petroleum processing; shale oil processing. It is used as an intermediate for dyes, fungicides, insecticides, herbicides, pharmaceuticals, plant growth hormones; 1,8 naphthalic acid; in the manufacture of some plastics; and has been detected in cigarette smoke and gasoline exhaust condensates; a constituent of coal tar creosote, asphalt, and diesel fuel. It has been used as an polyploidy agent.
운송 방법
UN3082 Environmentally hazardous substances, liquid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required.
Purification Methods
It has also been purified by chromatography from CCl4 on alumina with *benzene as eluent [McLaughlin & Zainal J Chem Soc 2485 1960]. [Beilstein 5 IV 1834.]
비 호환성
Ozone and strong oxidizing agents, including perchlorates, chlorine, fluorine, and bromine
폐기물 처리
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal. In accordance with 40CFR165, follow recommendations for the disposal of pesticides and pesticide containers. Must be disposed properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office. Incineration or permanganate oxidation
아세나프텐 준비 용품 및 원자재
원자재
준비 용품
1,4,5,8-나프탈렌테트라카르복실산
1-아미노-8-나프토익산
아세나프틸렌
1,8-나프탈산
fluorescent whitening agent EFR
Acenaphthenequinone
1,8-나프탈산 무수물
4,5-디클로로나프탈렌-1,8-디카르복실산무수물
5-니트로아세나프텐
4-브로모-1,8-나프탈산 무수물
Acenaphthylene, 1,2-dihydro-1-methoxy-
2a,3,4,5-테트라하이드로아세나프텐
5-bromoacenaphthylene-1,2-dione
3,9-페릴렌디카르복실산
데카사이클렌
1-아세나프테논