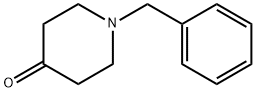
1-벤질-4-피페리돈 C화학적 특성, 용도, 생산
화학적 성질
Clear colorless to straw coloured oily liquid
용도
A substituted piperidine as pesticide
화학 반응
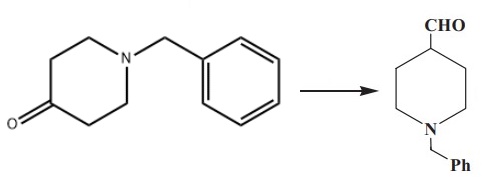
As an important organic compound, N-Benzyl-4-piperidone could be used to synthesize many organic compounds. There are four ways to synthesize 1-benzylpiperidine-4-carbaldehyde using it as the raw material.
The first method involves the Wittig reaction of (methoxymethyl) triphenylphosphonium chloride with N-benzyl-4-piperidone, followed by hydrolysis of the resulting enol ether.
In another method, trimethylsilyl diazomethane is condensed with N-benzyl-4-piperidone, followed by hydrolysis to obtain the final product.
In a third route, N-benzyl-4-piperidone is treated with trimethyloxosulfonium iodide to produce the corresponding epoxide. The epoxide is then rearranged in the presence of magnesium bromide etherate, resulting in 1-benzylpiperidine-4-carbaldehyde with high yields.
In 2007, a patent described the Darzens condensation of N-benzyl-4-piperidone with ethyl chloroacetate, followed by decarboxylation[1].
Synthesis
1-Benzyl-4-piperidone was synthesized with benzylamine and methyl acrylate as raw materials via 1,4-addition, Dieckmann condensation and hydrolysis decarboxylafion reactions. Add 150 mL of anhydrous toluene and 2.8 g of metallic sodium to a 250 mL dry three-necked flask, stir and heat to reflux. Add 1 mL of anhydrous methanol, then slowly drop 28 g of N.N-bis(β-propionate methyl ester) benzylamine. After adding N.N-bis(β-propionate methyl ester), benzylamine is completed, reflux for 6 h. During the reflux process, the stirring speed needs to be increased, and 100 ml of anhydrous toluene is added to the reaction vessel in batches. Stop reflux, cool to room temperature, extract the mixture with 150 mL of 25% (mass fraction) hydrochloric acid solution, and reflux in an oil bath for 5 h until there is no colour change in the FeCl3 solution test, indicating that the reaction is over. Cool the reaction mixture, add 35% NaOH solution while stirring to neutralize to about pH=8.5, extract with ethyl acetate (100 ml×3), combine the ethyl acetate layers, wash with saturated NaCl solution, and dry over anhydrous magnesium sulfate. Ethyl acetate was recovered by distillation, and the remaining material was distilled under reduced pressure to obtain 14.8 g of 1-benzyl-4-piperidone with a yield of 78.4%. The product was a light yellow oily liquid.
Purification Methods
If the physical properties show contamination, then dissolve it in the minimum volume of H2O, made strongly alkaline with aqueous KOH, extract it with toluene several times, dry the extract with K2CO3, filter, evaporate and distil the residue at high vacuum using a bath temperature of 160-190o, and redistil it. [Brookes & Walker J Chem Soc 3173 1957, Bolyard J Am Chem Soc 52 1030 1930.] The hydrochloride has m 159-161o (from Me2CO/Et2O), and the picrate has m 174-182o (from Me2CO/Et2O). [Grob & Brenneisen Helv Chim Acta 41 1184 1958, Beilstein 21/6 V 424.]
1-벤질-4-피페리돈 준비 용품 및 원자재
원자재
준비 용품
N-Boc-2-피페리돈
4,5,6,7-TETRAHYDRO-2-METHYLFURO[3,2-C]PYRIDINE
1-BOC-4-HYDROXY-4-(하이드록시메틸)-피페리딘
4-(3-트리푸오로메틸)페닐-4-피페리디놀
1-BENZYL-4-HYDROXY-4-(3-TRIFLUOROTOLYL)피페리디놀
1-Benzyl-4-hydroxypiperidine
(1-BENZYLPIPERIDIN-4-YLIDENE)아세트산에틸에스테르
1H-아제핀-4-카르복실산,헥사히드로-,메틸에스테르,(4S)-(9CI)
4-(1-피롤리디닐)피페리딘
1H-아제핀-4-카르복실산,헥사하이드로-,메틸에스테르,(4R)-(9CI)
2-옥사-8-아자스피로[4.5]데칸
메틸1-벤질-4-히드록시피페리딘-4-카르복실레이트
4-(2-아미노에틸)-1-벤질피페리딘
4-(4-FLUORO-PHENYL)-PIPERIDIN-4-OL염산염
4-메틸렌피페리딘
TERT-BUTYL 1-OXA-4,9-DIAZASPIRO[5.5]UNDECANE-4-CARBOXYLATE