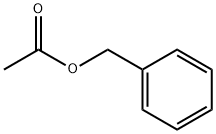
초산 벤질
|
|
초산 벤질 속성
- 녹는점
- -51 °C (lit.)
- 끓는 점
- 206 °C (lit.)
- 밀도
- 1.054 g/mL at 25 °C (lit.)
- 증기 밀도
- 5.1
- 증기압
- 23 mm Hg ( 110 °C)
- 굴절률
- n
20/D 1.502(lit.)
- 인화점
- 216 °F
- 저장 조건
- -20°C
- 용해도
- ≥35.7 mg/mL in EtOH; ≥49.4 mg/mL in DMSO
- 물리적 상태
- 액체
- 색상
- 무색 액체
- 냄새
- 달콤하고 꽃향기 나는 과일향
- 폭발한계
- 0.9-8.4%(V)
- ?? ??
- 꽃향기
- 생물학적 소스
- synthetic
- 수용성
- 23&C에서 <0.1g/100mL
- Merck
- 14,1123
- JECFA Number
- 23
- BRN
- 1908121
- 노출 한도
- ACGIH: TWA 10 ppm
- Dielectric constant
- 5.1(21℃)
- LogP
- 1.96 at 25℃
- 표면장력
- 37.3mN/m at 293.15K
- CAS 데이터베이스
- 140-11-4(CAS DataBase Reference)
- IARC
- 3 (Vol. 40, Sup 7, 71) 1999
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | Xi | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 36/37/38 | ||
| 안전지침서 | 26-37/39-24/25 | ||
| 유엔번호(UN No.) | 2810 | ||
| WGK 독일 | 1 | ||
| RTECS 번호 | AF5075000 | ||
| 자연 발화 온도 | 862 °F | ||
| TSCA | Yes | ||
| HS 번호 | 29153950 | ||
| 유해 물질 데이터 | 140-11-4(Hazardous Substances Data) | ||
| 독성 | LD50 orally in rats: 2490 mg/kg (Jenner) | ||
| 기존화학 물질 | KE-02778 |
초산 벤질 C화학적 특성, 용도, 생산
용도
음식 풍미, 매일 맛, 비누 풍미 etc.와 용매로 사용하는. 그것은 아로마 향과 향료에 사과 향과 배 향을 부여하기 위해 향수 및 화장품에 널리 사용됩니다. 벤질 아세테이트는 또한 플라스틱 및 수지, 셀룰로오스 아세테이트, 질산염, 오일, 래커, 광택제 및 잉크의 용매로 사용됩니다.순도시험
(1) 비중 : 이 품목의 비중은 1.052~1.056이어야 한다.
(2) 굴절률 : 이 품목의 굴절률 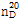 은 1.501~1.504이어야 한다.
은 1.501~1.504이어야 한다.
(3) 용상 : 이 품목 1mL를 60% 에탄올 5mL에 녹일 때, 그 액은 징명하여야 한다.
(4) 산가 : 이 품목의 산가는 향료시험법 중 산가측정법에 따라 시험할 때, 1 이하이어야 한다.
(5) 염소화합물 : 이 품목은 향료시험법 중 할로겐시험법의 동망법에 따라 시험할 때, 이에 적합하여야 한다.
확인시험
이 품목 1mL에 10% 알콜성수산화칼륨용액 5mL를 넣고 온탕중에서 20분간 가열하면 특이한 향기는 없어지고 식힌 다음 이에 묽은염산 1mL 및 물 8mL를 섞은 액은 확인시험법 중 초산염 (다)의 반응을 나타낸다.
정량법
이 품목 약 0.8g을 정밀히 달아 향료시험법 중 에스테르가 및 에스테르함량측정법에 따라 시험한다.
0.5N 알콜성수산화칼륨용액 1mL = 75.09mg C9H10O2
포장, 보관 및 운송
화기, 열 또는 기타 발화원 가까이에서 취급하거나 보관하지 마십시오. 용기를 닫아 두십시오. 용기는 조심해서 다루십시오. 가능한 압력 방출을 제어하기 위해 서서히여십시오. 시원하고 통풍이 잘되는 곳에 보관하십시오.물리적 성질
Benzyl acetate has a characteristic flowery (jasmine) odor and a bitter, pungent taste. It is the main component of jasmine absolute and gardenia oils. It occurs as a minor component in a large number of other essential oils and extracts. It is a colorless liquid with a strong, fruity, jasmine odor. In terms of volume, benzyl acetate is one of the most important fragrance and flavor chemicals. Although benzyl acetate is present in some essential oils at levels up to 65%, most of the commercial product is of synthetic origin.화학적 성질
Benzyl acetate is a colorless liquid with a fruity odor. On burning and decomposition, it produces irritating fumes. Benzyl acetate reacts with strong oxidants causing fire and explosion hazard.
Benzyl acetate is stable under normal conditions of use. Heating to decomposition may release carbon monoxide, carbon dioxide and other potentially toxic fumes and gases. Vapors may form explosive mixtures with air at elevated temperatures (> 90°C / 194°F). Avoid heat, open flames and other potential sources of ignition.
It has been used as a food additive in fruit flavours and as a component of perfumes since the early 1990s and is widely used as a fragrance in soaps, detergents and incense. There is widespread human exposure to benzyl acetate by ingestion, skin application and inhalation.
출처
Present as a main constituent in several oils and flower absolutes: ylang-ylang, cananga, neroli, jasmine, hyacinth, gardenia, tuberose. It has been isolated from the essential oil of the flowers of Loiseleuria procumbens Desv. (azelea). Also reported found in apricot, cooked asparagus, mozzarella cheese, grilled beef, cooked pork, malt whiskey, fresh mango, malt, wort and clams.용도
Benzyl acetate is used as an artificial jasmine and other perfumes, soap perfume, flavoring agent, solvent for cellulose acetate and nitrate, natural and synthetic resins, oils, lacquers, polishes, printing inks, and varnish removers.용도
Benzyl acetate is produced by reacting benzyl chloride with an alkali acetate or by esterifying benzyl alcohol with acetic anhydride. It is also formed in the oxidation of toluene in the presence of acetic acid or acetic anhydride. Benzyl acetate is one of the most used odorants. It is also used as a flavor and, to a small extent, as a high-boiling solvent.주요 응용
Benzyl acetate can be used as a high boiling point solvent in coatings, like ink coating, binding agent and paint remover. It is used in soap and other chemical essences and has the effect of promote in floral and fantasy essences in jasmine, white orchid, fragrant plantain lily, gekkakou, narcissus and other essences. It is a plasticizer for ionophore membranes.정의
ChEBI: Benzyl acetate is the acetate ester of benzyl alcohol. It has a role as a metabolite. It is an acetate ester and a benzyl ester.제조 방법
Benzyl acetate is produced by the interaction of benzyl chloride and sodiumacetate, by acetylation of benzyl alcohol, or from benzaldehyde and acetic acid with zinc dust.일반 설명
Colorless liquid with an odor of pears.공기와 물의 반응
Insoluble in water.반응 프로필
Benzyl acetate is an ester. Esters react with acids to liberate heat along with alcohols and acids. Strong oxidizing acids may cause a vigorous reaction that is sufficiently exothermic to ignite the reaction products. Heat is also generated by the interaction of esters with caustic solutions. Flammable hydrogen is generated by mixing esters with alkali metals and hydrides. Benzyl acetate is incompatible with strong oxidizing agents. Benzyl acetate is also incompatible with acids, bases and reducing agents.건강위험
Exposures to benzyl acetate cause adverse health effects. The symptoms of toxicity and poisoning include irritation to the skin, eyes, burning sensation, confusion, dizziness, drowsiness, labored breathing, sore throat, nausea, vomiting, and diarrhea. Benzyl acetate also causes adverse health effects to the respiratory tract and the CNS system with neurological effects.화재위험
Benzyl acetate is combustible.Safety Profile
A poison by inhalation.Moderately toxic by ingestion and subcutaneous routes.Human systemic effects by inhalation: an antipsychotic,unspecified respiratory and urinary system effects.Questionable carcinogen with experimental tumorigenicdata. CombustiCarcinogenicity
Not listed by ACGIH, IARC, NTP, or California Proposition 65.신진 대사
The esters of benzyl alcohol, such as the acetate, benzoate, cinnamate and hydrocinnamate, are rapidly hydrolysed in vivo to benzyl alcohol which is then oxidized to benzoic acid and excreted as hippuric acid저장
Benzyl acetate should be kept stored in a cool, dry place with the container closed when not in use.Purification Methods
Purify the acetate by fractional distillation, preferably in a good vacuum. Values of n25 of 1.5232-1.5242 are too high and should be nearer to 1.4994. [Merker & Scott J Org Chem 26 5180 1961, Beilstein 6 IV 2262.]주의 사항
Exposures to benzyl acetate far above the OEL may result in unconsciousness. After handling and using benzyl acetate, workers should wash thoroughly and remove contaminated clothing, washing it before reuse. Workers should avoid any kind of contact of benzyl acetate with the eyes, skin, ingestion, and inhalation. Workers should wear safety glasses and chemical goggles to avoid splashing of the chemical substance during work, and wear appropriate protective gloves and clothing to prevent skin exposure초산 벤질 준비 용품 및 원자재
원자재
아세트산 나트륨
염화벤질
염화칼슘
벤질 알코올
톨루엔
염소(기체)
이소 프로필 아세테이트
아세트산(빙초산)
오렌지꽃오일
Cinnamyl bromide
benzyl 2-acetyloxybenzoate
살리실산벤질
준비 용품
초산 벤질 공급 업체
글로벌( 759)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Wuhan Biet Co., Ltd. | 17320528784 +8617320528784 |
min@biet.com.cn | China | 42 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 |
sales1@chuanghaibio.com | China | 5251 | 58 |
| Hebei Kingfiner Technology Development Co.Ltd | +86-15532196582 +86-15373005021 |
lisa@kingfinertech.com | China | 3003 | 58 |
| Hebei Chuanghai Biotechnology Co., Ltd | +8617732866630 |
abby@chuanghaibio.com | China | 8773 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8618531123677 |
faithe@yan-xi.com | China | 5853 | 58 |
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 |
deasea125996@gmail.com | China | 2472 | 58 |
| Hebei Zhuanglai Chemical Trading Co.,Ltd | +8613343047651 |
admin@zlchemi.com | China | 3692 | 58 |
| Shandong Deshang Chemical Co., Ltd. | +86-0531-8875-2665 +8613153039501 |
info@deshangchem.com | China | 661 | 58 |
| JINING XINHE CHEMICAL CO., LTD | +8615318402391 |
sales@xinhepharma.com | China | 809 | 58 |
| Capot Chemical Co.,Ltd. | +8613336195806 |
sales@capot.com | China | 29731 | 60 |