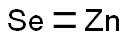셀레늄화 아연(셀렌화 아연)
|
|
셀레늄화 아연(셀렌화 아연) 속성
- 녹는점
- 1100 °C
- 밀도
- 5.42 g/mL at 25 °C(lit.)
- 용해도
- insoluble in H2O; soluble in dilute acid solutions
- 물리적 상태
- 불규칙한 플레이크
- 색상
- 노란색
- Specific Gravity
- 5.42
- 수용성
- 물에 불용성. 묽은 질산에서 분해됩니다.
- 감도
- Air Sensitive
- Merck
- 14,10156
- Solubility Product Constant (Ksp)
- pKsp: 25.44
- 노출 한도
- ACGIH: TWA 0.2 mg/m3
NIOSH: IDLH 1 mg/m3; TWA 0.2 mg/m3
- CAS 데이터베이스
- 1315-09-9(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | T,N | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 23/25-33-50/53 | ||
| 안전지침서 | 20/21-28-45-60-61 | ||
| 유엔번호(UN No.) | UN 3283 6.1/PG 2 | ||
| WGK 독일 | 3 | ||
| TSCA | Yes | ||
| 위험 등급 | 4.3 | ||
| 포장분류 | III | ||
| HS 번호 | 28429090 | ||
| 기존화학 물질 | KE-35579 | ||
| 유해화학물질 필터링 | 97-1-134 | ||
| 함량 및 규제정보 | 물질구분: 유독물질; 혼합물(제품)함량정보: 셀레늄 또는 그 화합물과 셀렌화합물을 1% 이상 함유한 혼합물 |
셀레늄화 아연(셀렌화 아연) C화학적 특성, 용도, 생산
개요
Zinc selenide (ZnSe) is an II-VI compound semiconductor material with a face-centred cubic crystal structure and excellent physicochemical properties, which has a wide range of transmittance, from 0.5 microns to 19 microns. Zinc selenide is a very good infrared material, mainly used in infrared optics and optoelectronics, and can be used to manufacture window materials, focusing lenses, beam splitters, prisms, and CO2 lasers. Zinc selenide is also commonly used as lenses and windows for CO2 lasers due to its good imaging and thermal shock properties.화학적 성질
Zinc selenide(ZnSe) is a yellow shiny fine powder that rarely occurs in nature. It is a chemically inert, non-hygroscopic and highly pure material largely found in the mineral stilleite. The transmissivity of zinc selenide is relatively constant over the wavelength range between 0.7 × 10 -6 to 16 × 10 -6 m, making it an excellent choice for flare radiation measurement. ZnSe is highly transparent to the radiation emitted from a flare flame. It is effective in many optical applications owing to its high resistance to thermal shock, stability in virtually all environments and extremely low bulk losses.
용도
Zinc selenide (ZnSe) is used as an infrared optical material with wide transmission wavelength range. It is also used as an entrance optic in the new range of "in-ear" clinical thermometers. ZnSe is used as a semiconductor material for thin film devices, and n-type windows layer for thin film heterojunction solar cells. ZnSe activated with tellurium is a scintillator suitable for matching with photodiodes. It is used in x-ray and gamma ray detectors. Zinc selenide films are useful in photovoltaic cells and solar conversion cells. The material can be doped with n-type and p-type doping. It is used to form light-emitting diodes and diode lasers. ZnSe doped with chromium finds use as an IR laser gain medium.공기와 물의 반응
ZnSe is insoluble in water, but reacts with acids to form toxic hydrogen selenide gas. It can be deposited as a thin film by chemical vapour deposition techniques including MOVPE and vacuum evaporation.위험도
Fire risk in contact with water or acids.Synthesis
Zinc Selenide is produced by synthesis from Zinc vapour and H2Se gas, forming as sheets on Graphite susceptors. Another method of producing is a growth from melt under excessive pressure of inert gas (Ar usually).참고 문헌
[1] HYUN SEON HONG. Facile synthesis and characterization of zinc selenide nanoparticles in aqueous solution at room temperature[J]. Journal of Crystal Growth, 2020, 535: Article 125523. DOI:10.1016/j.jcrysgro.2020.125523.[2] HILE D D, SWART H C, MOTLOUNG S V, et al. Zinc selenide semiconductor: synthesis, properties and applications[J]. Nanoscale Compound Semiconductors and their Optoelectronics Applications, 2022. DOI:10.1016/b978-0-12-824062-5.00001-4.
[3] https://www.crystran.co.uk/optical-materials/zinc-selenide-znse
셀레늄화 아연(셀렌화 아연) 준비 용품 및 원자재
원자재
준비 용품
셀레늄화 아연(셀렌화 아연) 공급 업체
글로벌( 119)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| career henan chemical co | +86-0371-86658258 +8613203830695 |
sales@coreychem.com | China | 29855 | 58 |
| City Chemical LLC | 2039322489 |
sales@citychemical.com | United States | 168 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 |
linda@hubeijusheng.com | CHINA | 28172 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 |
linda@hubeijusheng.com | CHINA | 22963 | 58 |
| Hubei Ipure Biology Co., Ltd | +8613367258412 |
ada@ipurechemical.com | China | 10244 | 58 |
| HONG KONG IPURE BIOLOGY CO.,LIMITED | 86 18062405514 18062405514 |
ada@ipurechemical.com | CHINA | 3461 | 58 |
| Dayang Chem (Hangzhou) Co.,Ltd. | +86-0571-88938639 +8617705817739 |
info@dycnchem.com | China | 52846 | 58 |
| Zhuoer Chemical Co., Ltd | 02120970332; +8613524231522 |
sales@zhuoerchem.com | China | 2904 | 58 |
| changzhou huayang technology co., ltd | +8615250961469 |
2571773637@qq.com | China | 9647 | 58 |
| LEAP CHEM CO., LTD. | +86-852-30606658 |
market18@leapchem.com | China | 24727 | 58 |
셀레늄화 아연(셀렌화 아연) 관련 검색:
카드뮴 텔루륨화합물 인화 인듐 황화아연 셀레늄화 아연(셀렌화 아연) Cadmium selenide, solid soln. with cadmium sulfide, zinc selenide and zinc sulfide, aluminum and copper-doped Cadmium selenide, solid soln. with cadmium sulfide, zinc selenide and zinc sulfide, europium doped Cadmium selenide, solid soln. with cadmium sulfide, zinc selenide and zinc sulfide, gold and manganese-doped Cadmium selenide, solid soln. with cadmium sulfide, zinc selenide and zinc sulfide, manganese and silver-doped Cadmium selenide, solid soln. with cadmium sulfide, zinc selenide and zinc sulfide, copper and manganese-doped
Zinc sulfate heptahydrate
Zinc silicofluoride
ZINC TELLURIDE
Zinc chloride
Zinc iodide
Zinc oxide
Zinc naphthenate
Zinc bis(trifluoromethylsulfonyl)imide
ZINC DIHYDROGEN PHOSPHATE