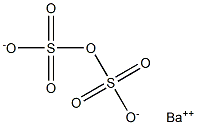
Barium Pyrosulfate
|
|
Barium Pyrosulfate 속성
안전
Barium Pyrosulfate C화학적 특성, 용도, 생산
개요
Barium pyrosulfate has the molecular formula of BaS2O7 and the molecular weight of 313.4578 g/mol. There is no data concerning this salt is available either in patents, scientific or technical literature, or in reference books. Indeed, it has been stated that “This salt does not existdit was first prepared by J.J. Berzelius in 1843 ..” “It is decomposed by water . Apart from some doubt as to its precise structure, the compound is of little interest, and has never been more than a chemical curiosityd1933”. However, this salt can be prepared by the solid-state reaction of ammonium pyrosulfate with barium chloride:BaCl2 + (NH4)2S2O7 + heat
? BaS2O7 + 2HCl(gas) + 2NH3(gas)
However, a better method of preparation involves reaction of the sulfate with fuming sulfuric acid (Oleum):
BaSO4 +H2SO4?BaS2O7 +H2O
The syrupy solution is heated to >150–175°C to drive off the water and allow crystals to appear. Once formed, barium pyrosulfate is stable to about 720°C where it decomposes to the sulfate plus sulfur trioxide gas. It can also be prepared by heating the persulfate:
2BaS2O8 + heat ? 2BaS2O7 + O2
The persulfate is also called peroxodisulfate. Further heating will form the sulfate, BaSO4 plus sulfur trioxide. It is expected that this salt will be unstable in water. However, the solubility of this salt has not been reported.
Barium Pyrosulfate 준비 용품 및 원자재
원자재
준비 용품
Barium Pyrosulfate 공급 업체
글로벌( 0)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|