Nitric acid suppliers
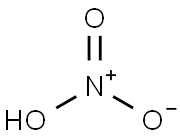
Nitric acid
- CAS:
- 7697-37-2
- MF:
- HNO3
- MW:
- 63.01
Properties
- Melting point:
- -42 °C
- Boiling point:
- 120.5 °C(lit.)
- Density
- 1.41 g/mL at 20 °C
- vapor density
- 1 (vs air)
- vapor pressure
- 8 mm Hg ( 20 °C)
- Flash point:
- 120.5°C
- storage temp.
- Store at +2°C to +25°C.
- solubility
- Miscible with water.
- pka
- -1.3(at 25℃)
- form
- Liquid, Double Sub-Boiling Quartz Distillation
- Specific Gravity
- d 20/4 1.4826
- color
- colorless to deep yellow
- PH Range
- 1
- Odor
- Suffocating fumes detectable at <5.0 ppm
- PH
- 3.01(1 mM solution);2.04(10 mM solution);1.08(100 mM solution);
- Water Solubility
- >100 g/100 mL (20 ºC)
- Sensitive
- Hygroscopic
- Merck
- 14,6577
- Exposure limits
- TLV-TWA 2 ppm (5 mg/m3) (ACGIH, MSHA, OSHA, and NIOSH); STEL 4 ppm (~10 mg/m3) (ACGIH).
- Dielectric constant
- 55.0(14℃)
- LogP
- -0.773 (est)
- CAS DataBase Reference
- 7697-37-2(CAS DataBase Reference)
- NIST Chemistry Reference
- Nitric acid(7697-37-2)
- EPA Substance Registry System
- Nitric acid (7697-37-2)
Safety Information
- Symbol(GHS)



GHS03,GHS05,GHS06
- Signal word
- Danger
- Hazard statements
- H272-H290-H314-H331
- Precautionary statements
- P210-P220-P280-P303+P361+P353-P304+P340+P310-P305+P351+P338
- Hazard Codes
- C,O,Xi,T+
- Risk Statements
- 8-35-34-20-41-37/38-36/38-26/27
- Safety Statements
- 23-26-36-45-36/37/39-39-60-28
- OEB
- B
- OEL
- TWA: 2 ppm (5 mg/m3), STEL: 4 ppm (10 mg/m3)
- RIDADR
- UN 3264 8/PG 3
- WGK Germany
- 1
- RTECS
- QU5900000
- F
- 8
- TSCA
- Yes
- HS Code
- 2808 00 00
- HazardClass
- 8
- PackingGroup
- II
- Hazardous Substances Data
- 7697-37-2(Hazardous Substances Data)
- Toxicity
- LC50 inhal (rat)
2500 ppm (1 h)
PEL (OSHA)
2 ppm (5 mg/m3)
TLV-TWA (ACGIH)
2 ppm (5.2 mg/m3)
STEL (ACGIH)
4 ppm (10 mg/m3)
- IDLA
- 25 ppm
Use
Nitric acid (HNO3) is an important industrial acid used to alter or produce many products such as fertilizers and explosives. It reacts with ammonia to produce ammonium nitrate, an important commercial chemical. It is used for nitrating numerous other compounds to produce nitrates. Nitricacid is used to produce adipic acid (C6H4O10), which is used in the production of nylon (seeNylon). Additional uses of nitric acid are for oxidation, nitration, and as a catalyst in numerousreactions. Salts of nitric acid are collectively called nitrates, which are soluble in water. Nitricacid is used in the production of many items such as dyes, pharmaceuticals, and syntheticfabrics.It is also used in a variety of processes including print making.
According to relevant laws, regulations, policies and the provisions of this website, this website does not display the product information!
The source is "易制爆、炸药、化学武器等". If the information is inaccurate, please contact the platform to modify it.
contact information:info@chemicalbook.com 400-158-660