简介
1-(2,2-二氟苯并[D][1,3]二氧杂环戊烯-5-基)环丙烷甲酸是近年来发展较快的一种新型饲料添加剂,属于酸化剂的一种。添加到动物饲料中具有降低肠道pH、调节肠道微生态平衡、提高饲料营养物质的消化和吸收等功能[1-2]。另有研究报道,1-(2,2-二氟苯并[D][1,3]二氧杂环戊烯-5-基)环丙烷甲酸能通过微生物细胞膜,在其细胞内分解产生H+,降低或抑制细胞内酶的活性,破坏微生物蛋白和核酸的营养代谢,从而使微生物不能繁殖,甚至死亡[3-4]。1-(2,2-二氟苯并[D][1,3]二氧杂环戊烯-5-基)环丙烷甲酸盐阴离子在细胞壁外分解细菌细胞壁蛋白质,发挥杀菌和抑菌作用[5]。1-(2,2-二氟苯并[D][1,3]二氧杂环戊烯-5-基)环丙烷甲酸的抗菌作用减少了微生物的数量,尤其是大肠杆菌等致病菌的数量,减少了微生物代谢物的产生,从而减少了对肠道的损害[6]。
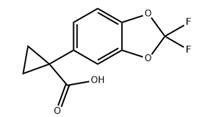
图1 1-(2,2-二氟苯并[D][1,3]二氧杂环戊烯-5-基)环丙烷甲酸的结构式。
合成

图2 1-(2,2-二氟苯并[D][1,3]二氧杂环戊烯-5-基)环丙烷甲酸的合成路线[7]。
将化合物19(1当量)的DMSO溶液(1.25卷)添加到NaCN(1.4当量)的DMSO溶液中,保持温度在30-40℃之间。混合物搅拌1小时,然后加入水,然后加入MTBE。搅拌30分钟后,层分离。水层用MTBE提取。结合的有机层用水洗涤,干燥(Na2SO4),过滤和浓缩,以获得直接用于下一步的粗化合物20(95%)。产量100%。然后化合物20 (1.0 eq)、50 wt % KOH水溶液(5.0 eq) 1-溴-2-氯乙烷(1.5 eq)和Oct4NBr (0.02 eq)的混合物在70℃下加热1 h。将反应混合物冷却,然后与MTBE和水混合。有机相用水和盐水洗涤,然后除去溶剂,得到化合物21。产量100%。最后化合物21用6 M NaOH(8等当量)在乙醇(5卷)中在80°C水解。过夜。混合物冷却到室温,乙醇在真空下蒸发。将残渣放入水和MTBE中,加入1 M HCl,层分离。然后用二环己胺(0.97当量)处理MTBE层。料浆冷却至0°C。,用庚烷过滤洗涤,得到相应的DCHA盐。将盐放入MTBE和10%柠檬酸中搅拌,直到所有固体溶解。将层分离,用水和盐水冲洗MTBE层。溶剂交换到庚烷,然后过滤,在真空烘箱中50°C干燥后得到化合物1-(2,2-二氟苯并[D][1,3]二氧杂环戊烯-5-基)环丙烷甲酸。产量69%。合成路线如图2所示。
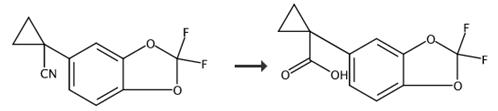
图3 1-(2,2-二氟苯并[D][1,3]二氧杂环戊烯-5-基)环丙烷甲酸的合成路线[8]。
在80°C下,用6 M NaOH(8当量)在乙醇(5体积)中水解化合物21。在夜间将混合物冷却至室温,并在真空下蒸发乙醇。将残留物放入水中,加入MTBE、1M HCl并分离各层。然后用二环己胺(0.97当量)处理MTBE层。将浆料冷却至0°C。,过滤并用庚烷洗涤,得到相应的DCHA盐。将盐放入MTBE和10%柠檬酸中,搅拌直至所有固体溶解。分离各层,用水和盐水洗涤MTBE层。在50°C的真空烘箱中干燥后,溶剂交换为庚烷,然后过滤得到化合物1-(2,2-二氟苯并[D][1,3]二氧杂环戊烯-5-基)环丙烷甲酸。收率69%。合成路线如图3所示。
应用
许多研究表明在仔猪饲粮中添加1-(2,2-二氟苯并[D][1,3]二氧杂环戊烯-5-基)环丙烷甲酸能改善仔猪的日增重,提高饲料转化效率[9]。研究表明,在仔猪饲粮中添加1-(2,2-二氟苯并[D][1,3]二氧杂环戊烯-5-基)环丙烷甲酸1%与对照组比日增提高7.1%,料肉比降低13.71%,稍影响采食量[10]。在生长仔猪饲粮中添加 1-(2,2-二氟苯并[D][1,3]二氧杂环戊烯-5-基)环丙烷甲酸1.8%,日增重提高8%~18%。1-(2,2-二氟苯并[D][1,3]二氧杂环戊烯-5-基)环丙烷甲酸在家禽方面的应用报道较少[11]。有研究者在肉仔鸡饲粮中添不同水平的1-(2,2-二氟苯并[D][1,3]二氧杂环戊烯-5-基)环丙烷甲酸,能显著增加肉仔鸡的采食量,提高饲粮的表观消化率和氮的沉积,日增重也有上升的趋势。添加量为0.6%时效果最明显,采食量和日增重分别提高了8.7%和5.8%,但饲料转化率差异不显著[12-13]。
参考文献
[1] R.J. Altenbach, A. Bogdan, S. Couty, N. Desroy, G.A. Gfesser, C.G. Housseman, P.R. Kym, B. Liu, T.T.T. Mai, K.F. Malagu, N. Merayo Merayo, O.L. Picolet, M.R. Pizzonero, X.B. Searle, S.E. Van der Plas, X. Wang, M.C. Yeung, Preparation of substituted N-sulfonylcyclopropanecarboxamides as modulators of the cystic fibrosis transmembrane conductance regulator protein, Galapagos NV, Belg.; AbbVie S.A.R.L.; Charles River Discovery Research Services UK Limited; Galapagos SASU . 2019, p. 189pp.
[2] K. Amala, G.V. Krishna, A. Ankamanayudu, G. Srinivasulu, K.D. Prasad, M.P. Reddy, N.V. Chowdary, Improved process for the preparation of cabozantinib and its pharmaceutically acceptable salts thereof, Natco Pharma Limited, India . 2019, p. 26pp.; Chemical Indexing Equivalent to 172:67847 (IN).
[3] A.D. Casarez, C.A. Coburn, T.A. Kellar, D.J. Buzard, N. Arora, Preparation of oxime compounds useful as T-cell activators and inhibitors of diacylglycerol kinases for the treatment and prevention of diseases, Gossamer Bio Services, Inc., USA . 2021, p. 197pp.
[4] B. Chan, B. Daniels, J. Drobnick, L. Gazzard, T. Heffron, M. Huestis, J. Liang, S. Malhotra, R. Mendonca, N. Rajapaksa, M. Siu, C. Stivala, J. Tellis, W. Wang, B. Wei, A. Zhou, M.W. Cartwright, E. Gancia, G. Jones, M. Lainchbury, A. Madin, E. Seward, D. Favor, K.C. Fong, Y. Hu, A. Good, B. Hu, A. Lu, Preparation of naphthyridine compounds as inhibitors of HPK1 useful for treatment of cancer, Genentech, Inc., USA; F. Hoffmann-La Roche AG . 2018, p. 928pp.
[5] C. Chen, S. Feng, K.S. Chan, Rhodium Porphyrin Catalyzed Regioselective Transfer Hydrogenolysis of C-C σ-Bonds in Cyclopropanes with iPrOH, Organometallics 38(12) (2019) 2582-2589.
[6] C. Hardouin, A. Poixblanc, F. Bariere, R. Tamion, T. Dubuffet, Y. Hervouet, P. Mouchet, Development of an Efficient Synthesis of an Agonist of Acetylcholine Nicotinic Receptor, Org. Process Res. Dev. 22(10) (2018) 1419-1425.
[7] T.M. Kapoor, Preparation of pyrazoloquinazolinones as antitumor agents, USA . 2018, p. 93pp.
[8] S. Kaul, N. Alkayed, S. Nagarajan, A. Cianciulli, F. Micheli, T. Semeraro, I.M.L. Trist, Preparation piperazine derivatives and related heterocycles as antagonists of GPR39 protein for the treatment of diseases, Oregon Health & Science University, USA . 2021, p. 376pp.
[9] J.K. Lohmann, I.R. Craig, T.A. Stoesser, M. Semar, M. Fehr, B. Mueller, W. Grammenos, T. Grote, M. Seet, Synthesis of [1,2,4]triazole fungicides as seed coating for combating phytopathogenic fungi, BASF SE, Germany . 2021, p. 115pp.
[10] L. McLaren, D. To, D. Tovell, S. Abele, Process for the synthesis of 1-aryl-1-trifluoromethylcyclopropanes, Idorsia Pharmaceuticals Ltd., Switz. . 2018, p. 31 pp.
[11] S. Patel, G. Hamilton, G. Zhao, H. Chen, B. Daniels, C. Stivala, Preparation of bicyclic ketones for the treatment of diseases, F. Hoffmann-La Roche AG, Switz.; Genentech, Inc. . 2019, p. 358pp.
[12] A. Pesyan, M.F. Balandrin, Preparation of β-phenylalkanamide derivatives useful for treatment of central nervous system diseases and disorders, Aurimmed Pharma, Inc., USA . 2021, pp. 87 pp., Cont.-in-part of U.S. Ser. No. 938,341.
[13] G. Srinivasulu, A. Ankamanayudu, G.V. Krishna, K. Amala, K.D. Prasad, M.P. Reddy, N.V. Chowdary, Improved process for the preparation of cabozantinib and its pharmaceutically acceptable salts thereof, Natco Pharma Limited, India . 2019, p. 34pp.; Chemical Indexing Equivalent to 172:74489 (WO).
