| Company Name: |
|
| Tel: |
|
| Email: |
amit.singh@adventchembio.com |
| Products Intro: |
Cas:10125-13-0
ProductName:Copper(II) chloride dihydrate (Cupric chloride dihydrate)
Purity: 98% | Package: 1 kg,5 kg, 10 kg,25kg and 1 MT
|
|
| Product Name: | Copper Chloride Dihydrate | | Synonyms: | COPPER (II) CHLORIDE, HYDROUS;COPPER(+2)CHLORIDE DIHYDRATE;Copper(II)chloridedihydrate(99.999%-Cu)PURATREM;Copper(II)chloridedihydrate,99+%(ACS);Copper chloride (CuCl2), dihydrate;copper(ii) chloride dihydrate, acs;copper(ii) chloride hydrate, puratronic;CUPRICCHLORIDE,DIHYDRATE,CRYSTAL,PURIFIED | | CAS: | 10125-13-0 | | MF: | Cl2CuH2O | | MW: | 152.46 | | EINECS: | 600-176-4 | | Product Categories: | metal halide | | Mol File: | 10125-13-0.mol | 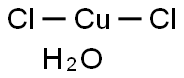 |
| | Copper Chloride Dihydrate Chemical Properties |
| Melting point | 100 °C (dec.)(lit.) | | density | 2.54 | | vapor density | >1 (vs air) | | storage temp. | Store at +5°C to +30°C. | | solubility | 757g/l | | form | Solid | | Specific Gravity | 2.54 | | color | White | | PH | 3.0-3.8 (50g/l, H2O, 20℃) | | Water Solubility | 1150 g/L | | Sensitive | Air Sensitive & Hygroscopic | | Merck | 14,2633 | | Exposure limits | ACGIH: TWA 1 mg/m3
NIOSH: IDLH 100 mg/m3; TWA 1 mg/m3 | | Stability: | Reacts violently with potassium, sodium. Contact with acetylene can form shock-sensitive acetylides. Hygroscopic - incompatible with moisture. | | InChIKey | ORTQZVOHEJQUHG-UHFFFAOYSA-L | | LogP | -1.380 (est) | | CAS DataBase Reference | 10125-13-0(CAS DataBase Reference) | | EPA Substance Registry System | Cupric chloride dihydrate (10125-13-0) |
| | Copper Chloride Dihydrate Usage And Synthesis |
| Description | Copper (II) chloride dihydrate is used in the preparation of copper oxychloride and as a catalyst in many organic chlorination reactions such as vinyl chloride and 1,2-dichloroethane. Copper chloride dihydrate may be also used as catalyst to cleave tetrahydropyranyl (THP) ethers and t-butyldimethylsilyl (TBDMS) ethers and to chemoselectively hydrolyze semicarbazones to carbonyl compounds. Solution of copper (II) chloride is used for plating on aluminum and for tinting germanium and tin.
| | Uses | Copper(II) chloride is used as a mordant in dyeing and printing of fabrics; as an ingredient of isomerization and cracking catalysts; and as a desulfurizing and deodorizing agent in petroleum industry.
Other important applications are in copper plating of aluminum; preparation of copper standard solutions; test for molybdenum; in tinting-baths for iron and tin; in pigments for ceramics and glasses; as a fixer and desensitizer reagent in photography; in mercury extraction from ores; in laundry-marking and invisible inks; and in manufacture of several copper salts.
| | Preparation | Copper(II) chloride may be synthesized by heating elemental copper with chlorine:
Cu + Cl2 CuCl2
Alternatively, it may be prepared by treating copper carbonate with hydrochloric acid followed by crystallization:
CuCO3 + 2HCl → CuCl2 + CO2 + H2O
In the above preparation, the hydrate of the salt crystallizes, precipitates, and may be dehydrated by heating under vacuum.
| | References | [1] H. Wayne Richardson, Handbook of Copper Compounds and Applications, 1997
[2] Ram N. Ram and Kiran Varsha, Regeneration of carbonyl compounds from semicarbazones by copper (II) chloride dihydrate, Tetrahedron Letters, 1991, vol. 32, 5829-5832
[3] Z. P. Tan, L. Wang, J. Bo. Wang, Deprotection of t- Butyldimethylsiloxy (TBDMS) Protecting Group with Catalytic Copper (II) Chloride Dihydrate, Chinese Chemical Letters, 2000, vol. 11, 753-756
| | Chemical Properties | Moist blue crystals. | | Uses | Copper(II) chloride (CuCl2) or cupric chloride, is a brownish-yellow hygroscopic powder,
or it may be formed as green deliquescent crystals. It is used in the dyeing and printing of
textiles, as a disinfectant, as red pigment in the glass and ceramic industries, and for green-colored
pyrotechnics, wood preservative, fungicide, deodorizer, water purification, feed additive,
and electroplating baths. | | Uses | Supplement (trace mineral). | | Uses | Copper(II) chloride dihydrate has been used as a positive control in dispersion of zebrafish embryo. It has also been used for the preparation of trace metals mixture. | | Definition | ChEBI: Copper(II) chloride dihydrate is a hydrate that is the dihydrate form of copper(II) chloride. It occurs naturally as the mineral eriochalcite. It has a role as an EC 5.3.3.5 (cholestenol Delta-isomerase) inhibitor. It is a hydrate and a halide mineral. It contains a copper(II) chloride. |
| | Copper Chloride Dihydrate Preparation Products And Raw materials |
|