| Company Name: |
HUNAN CHEMFISH SCIENTIFIC CO.,LTD
|
| Tel: |
0731-+86-0731-85567275 18932438858 |
| Email: |
sales05@chemfish.com |
| Products Intro: |
Cas:1317-61-9
ProductName:Iron oxide
Purity: 99% | Package: 5KG;1KG
|
| Company Name: |
Shanghai Tengjun Biotechnology Co. LTD
|
| Tel: |
19821570922 |
| Email: |
zhanghongwei@labgogo.com |
| Products Intro: |
Cas:1317-61-9
ProductName:Iron oxide(II,III), magnetic nanoparticles
Purity: 99% 50nm | CustNote: 100g\500g\25kg
|
| Company Name: |
Meryer (Shanghai) Chemical Technology Co., Ltd.
|
| Tel: |
18621169109 |
| Email: |
market03@meryer.com |
| Products Intro: |
Cas:1317-61-9
ProductName:Iron oxide(II,III), magnetic nanoparticles solution
Purity: 10-30 nm, 25% in H2O | Package: 100ml;2.5l;25ml;500ml | CustNote: M84587
|
| Company Name: |
Nanjing Wogni Chemical Co., Ltd.
|
| Tel: |
15996452985 |
| Email: |
foxerhy@163.com |
| Products Intro: |
Cas:1317-61-9
ProductName:Iron oxide (Fe3O4)
Purity: 98% | Package: 10g;100g;1kg;10kg;100kg
|
- Iron oxide
-

- $0.00 / 25KG
-
2025-03-21
- CAS:1332-37-2
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 50000KG/month
- Iron oxide
-

- $0.10 / 1KG
-
2024-08-21
- CAS:1332-37-2
- Min. Order: 1KG
- Purity: 99.0%
- Supply Ability: 1000Tons
|
| Product Name: | Iron Oxide Magnetic | | Synonyms: | Iron(II,III) oxide, 98% (metals basis);Iron(II,III) oxide, 97% (metals basis);Iron(II,III) oxide, 99.95% (metals basis);Iron(II,III) oxide: (Ferrosoferric oxide: Iron(III) oxide);Iron (II, III) oxide, 99.5% min (metals basis);Iron (II,III) oxide, 96% (metals basis) Synthetic black iron oxide;Iron(II,III) oxide, Puratronic, 99.997% (metals basis);Iron oxide, magnetic nanoparticles solution | | CAS: | 1317-61-9 | | MF: | Fe3O4-2 | | MW: | 231.53 | | EINECS: | 215-277-5 | | Product Categories: | nano structured material;metal oxide;Inorganics | | Mol File: | 1317-61-9.mol | 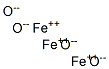 |
| | Iron Oxide Magnetic Chemical Properties |
| Melting point | 1538 °C(lit.) | | density | 4.8-5.1 g/mL at 25 °C(lit.) | | bulk density | 0.84g/mL | | refractive index | 3.0 | | Fp | 7 °C | | storage temp. | -20°C | | solubility | Aqueous Acid (Slightly) | | form | powder | | color | black | | Specific Gravity | 5.2 | | Odor | at 100.00?%. odorless | | Water Solubility | Insoluble in water and organic solvents. Soluble in concentrated mineral acids. | | Crystal Structure | Inverse spinel type | | crystal system | Cube | | Merck | 14,4040 | | Space group | Fd3m | | Lattice constant | | a/nm | b/nm | c/nm | α/o | β/o | γ/o | V/nm3 | | 0.83941 | 0.83941 | 0.83941 | 90 | 90 | 90 | 0.5915 |
| | Stability: | Stable. Incompatible with strong acids, chloroformates, peroxides. | | CAS DataBase Reference | 1317-61-9(CAS DataBase Reference) | | NIST Chemistry Reference | Iron(ii,iii) oxide(1317-61-9) | | EPA Substance Registry System | Iron oxide (Fe3O4) (1317-61-9) |
| Hazard Codes | Xi | | Risk Statements | 36/37/38 | | Safety Statements | 26 | | RIDADR | 3 | | WGK Germany | - | | TSCA | Yes | | HS Code | 28211000 |
| | Iron Oxide Magnetic Usage And Synthesis |
| Physical Properties | Black cubic crystal or amorphous powder; refractive index 2.42; density 5.17 g/cm3; Moh’s hardness 6.0; melts at 1,597°C; insoluble in water, soluble in acids.
| | Occurrence and Uses | Triiron tetroxide occurs in nature as the mineral magnetite, the magnetic oxide of iron. This mineral along with hematite is used as the starting material for producing iron, steel and other ferro-alloys.
| | Preparation | Triiron tetroxide is obtained from its natural mineral magnetite. In the laboratory the compound may be prepared by adding sodium hydroxide solution to an aqueous solution of 1:2 molar mixture of ferrous and ferric salt. (i.e., 1 mol FeCl2 + 2 mol FeCl3). The resulting black precipitate of the hydroxide on heating dehydrates to gives triiron tetroxide:
Fe2+(aq) + 2Fe3+(aq) + 8OH¯(aq) → Fe3(OH)8(s)
Fe3(OH)8(s) → Fe3O4(s) + 4H2O(g)
Also, the tetroxide may be produced by partial oxidation of iron or iron(II) sulfate by heating under limited amount of air. Another method of production involves heating iron metal with steam:
3Fe + 4H2O → Fe3O4 + 4H2
| | Reactions | Triiron tetroxide, when heated at elevated temperatures with a reducing agent such as hydrogen or carbon monoxide in the absence of air, produces metallic iron:
Fe3O4 + 4H2 → 3Fe + 4H2O
Partial reduction gives iron(II) oxide. When treated with concentrated acids, the tetroxide dissolves in acids forming mixtures of iron(II) and iron(III) salts:
Fe3O4 + 8HCl → FeCl2 + 2FeCl3 + 4H2O
Fe3O4 + 4H2SO4 → FeSO4 + Fe2(SO4)3 + 4H2O
| | Chemical Properties | Magnetite (Fe3O4) constitutes the richest ore
of iron; it is black and magnetic, one variety being called "lodestone". | | Chemical Properties | solid | | Uses | Iron(II,III) oxide (Fe3O4) can be used as a heterogeneous catalyst for the Fenton type oxidation of rhodamine B. It can be used as an anode material for the fabrication of lithium-ion batteries. Fe3O4 can also be utilized in the catalysis of the oxygen reduction reaction (ORR) in the anion exchange membrane fuel cell. | | Uses | Mostly it is used as a black- pigment -catalyst in the Haber process and in the water gas shift reaction. It is used as a contrast-agent in MRI scanning, whereas it is used as a pigment in magnetic applications, polishing compounds, cosmetics, medicines, polymer, rubber, filler, building, construction, appliances, and magnetic inks. | | Uses | Triiron tetraoxide can be used as pigment in paints, linoleum, ceramic glazes; in coloring glass; as a polishing compound; in the textile industry; in cathodes; as catalyst. | | General Description | Concentration 5mg/ml includes total weight nanocrystals plus ligands. | | Flammability and Explosibility | Non flammable | | Properties and Applications |
|
TEST ITEMS
|
SPECIFICATION
|
|
APPEARANCE
|
BLACK POWDER
|
|
CONTENT OF
Fe
3
O
4
|
90% min
|
|
pH VALUE
|
5-8
|
|
DENSITY
|
4.5 g/ml
|
|
SHADE
|
CLOSE TO STANDARD
|
|
OIL ABSORPTION
|
10-20%
|
|
RESIDUE ON 320 MESH
|
0.3% max
|
|
WATER SOLUBLE
|
0.3% max
|
|
VOLATITE 105 °C
|
1.0% max
|
|
TINTING STRENGTH
|
98-102 %
|
|
| | Iron Oxide Magnetic Preparation Products And Raw materials |
|