- Chromic sulfate
-

- $1.00 / 1KG
-
2025-04-23
- CAS:10101-53-8
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 10 mt
- Terlipressin Acetate
-

- $0.00 / 25KG
-
2025-03-21
- CAS:10101-53-8
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 50000KG/month
- Chromic sulfate
-

- $0.00 / 25KG
-
2025-03-21
- CAS:10101-53-8
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 50000KG/month
|
| Product Name: | Chromic sulfate | | Synonyms: | CHROMAL(TM);CHROMIC SULFATE;CHROMIC SULPHATE;CHROMIUM(+3)SULFATE;baychroma;baychromf;c.i.77305;chromicsulfate(cr2(so4)3) | | CAS: | 10101-53-8 | | MF: | Cr2O12S3 | | MW: | 392.18 | | EINECS: | 233-253-2 | | Product Categories: | Dye;Inorganics | | Mol File: | 10101-53-8.mol | 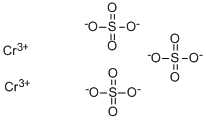 |
| | Chromic sulfate Chemical Properties |
| Provider | Language |
|
ALFA
| English |
| | Chromic sulfate Usage And Synthesis |
| Physical Properties | Reddish-brown hexagonal crystal; the pentadecahydrate is a dark green amorphous substance while the octadecahydrate is a violet cubic crystal; the densities are 3.10 g/cm3 (the anhydrous salt), 1.87 g/cm3 (pentadecahydrate), 1.709/cm3 (octadecahydrate); the anhydrous sulfate is insoluble in water and acids; the hydrate salts are soluble in water; the pentadecahydrate is insoluble in alcohol, but the octadecahydrate dissolves in alcohol.
| | Uses | Chromium(III) sulfate is used as the electrolyte for obtaining pure chromium metal. It is used for chrome plating of other metals for protective and decorative purposes. Other important applications of this compound are as a mordant in the textile industry; in tanning leather; to dissolve gelatin; to impart green color to paints, varnishes, inks, and ceramic glazes; and as a catalyst.
| | Preparation | Chromium(III) sulfate is prepared by treating chromium(III) hydroxide with sulfuric acid followed by crystallization:
2Cr(OH)3 + 3H2SO4 → Cr2(SO4)3 + 6H2O
| | Description | Chromic sulfate is a peach-colored solid or redto violet, odorless powder. Molecular weight =392.18.Hazard Identification (based on NFPA-704 M RatingSystem): Health 1, Flammability 0, Reactivity 0. Insolublein water | | Chemical Properties | Also known as chromic sulfate,Cr2(S04)3, is a violet or red powder that is insoluble in water and acids.The basic form (reduction of sodium dichromate) is used in tanning. Other uses are chrome plating,chromium alloys,mordant,catalyst,green paints and varnishes,green ink,and ceramic glazes. | | Chemical Properties | Chromic sulfate is a peach colored solid or red to violet, odorless powder. | | Uses | Chrome plating, chromium alloys, mordant,
catalyst, green paints and varnishes, green ink,
ceramics (glazes). The basic form (reduction of
sodium dichromate) is used in tanning. | | Definition | ChEBI: A compound of chromium and sulfate in which the ratio of chromium (in the +3 oxidation state) to sulfate is 2:3 | | General Description | Dark green to violet crystalline material. Used in paints and inks, ceramics, and in textile dyeing. I Chromic sulfate is noncombustible. The primary hazard of Chromic sulfate is the potential for environmental damage if released. Immediate steps should be taken to limit its spread to the environment. | | Air & Water Reactions | Water soluble. | | Reactivity Profile | Chromic sulfate has weak oxidizing or reducing powers. Redox reactions can however still occur. The majority of compounds in this class are slightly soluble or insoluble in water. If soluble in water, then the solutions are usually neither strongly acidic nor strongly basic. These compounds are not water-reactive. | | Health Hazard | INHALATION: Corrosive action on mucous membranes. SKIN: May elicit an allergic reaction. Corrosive action on skin. Lesions confined to exposed parts. | | Fire Hazard | Special Hazards of Combustion Products: Decomposes to chromic acid when heated. | | Potential Exposure | This compound is used in green paints, inks, dyes, and ceramics | | First aid | If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit | | storage | Color Code—Green: General storage may be used.Prior to working with chromic sulfate you should be trainedon its proper handling and storage. Store in tightly closedcontainers in a cool, well-ventilated area away from strongoxidizers and sources of heat. | | Shipping | UN3077 Environmentally hazardous substances, solid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required | | Incompatibilities | This chemical has weak oxidizing and reducing properties. Keep away from strong oxidizers Chromic Sulfate 837 (perchlorates, peroxides, permanganates, chlorates, nitrates, chlorine, bromine, and fluorine). When heated this chemical decomposes to chromic acid. | | Waste Disposal | Return to supplier where possible. Where this is not practical, the material should be encapsulated and buried in a specially designated chemical landfill. |
| | Chromic sulfate Preparation Products And Raw materials |
|