- Sodium tungstate
-

- $30.00 / 1KG
-
2025-04-15
- CAS:13472-45-2
- Min. Order: 50KG
- Purity: 99%
- Supply Ability: 500000kg
- TUNGSTEN
-

- $46.80 / 1KG
-
2025-03-26
- CAS:13472-45-2
- Min. Order: 1ASSAYS
- Purity: 99%
- Supply Ability: 100kg
|
| | Sodium tungstate Basic information |
| Product Name: | Sodium tungstate | | Synonyms: | AMMONIUM TUNGSTATE (PARA);SODIUM O-TUNGSTATE;SODIUM PARA-TUNGSTATE;W HC 40;TUNGSTEN AA SINGLE ELEMENT STANDARD;TUNGSTEN, AAS STANDARD SOLUTION;TUNGSTEN AA STANDARD;TUNGSTEN ATOMIC ABSORPTION STANDARD | | CAS: | 13472-45-2 | | MF: | Na2WO4 | | MW: | 293.82 | | EINECS: | 236-743-4 | | Product Categories: | Inorganics | | Mol File: | 13472-45-2.mol | 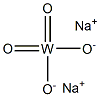 |
| | Sodium tungstate Chemical Properties |
| Melting point | 3410 °C(lit.) | | Boiling point | 5660 °C(lit.) | | density | 2.3 g/mL at 25 °C(lit.) | | vapor pressure | 0Pa at 25℃ | | solubility | H2O: soluble | | pka | 3.5[at 20 ℃] | | form | wire | | color | white rhombohedral crystals, crystalline | | Water Solubility | g/100g H2O: 71.5 (0°C), 73.0 (20°C), 97.2 (100°C) [LAN05] | | Merck | 13,8772 | | LogP | 0 | | CAS DataBase Reference | 13472-45-2(CAS DataBase Reference) | | EPA Substance Registry System | Sodium tungsten oxide (Na2WO4) (13472-45-2) |
| | Sodium tungstate Usage And Synthesis |
| Chemical Properties | Sodium tungstate is a sodium salt of orthotungstate. It is a colorless crystals or white crystalline powder. Soluble in water; insoluble in alcohol and acids. noncombustible. Sodium tungstate has three crystalline forms, δ, γ and β. The transition temperature from δ to γ is about 570℃, and from γ to β is about 585℃. In hydrogen is heated to 700 ℃ also does not change, to 1100 ℃ will become metal tungsten. | | Uses | Intermediate in preparation of tungsten compounds, (e.g., phosphotungstate), reagent, fire-proofing fabrics and cellulose, alkaloid precipitant. | | Definition | ChEBI: Sodium tungstate is an inorganic sodium salt having tungstate as the counterion. Combines with hydrogen peroxide for the oxidation of secondary amines to nitrones. It has a role as a reagent. It contains a tungstate. | | Preparation | Sodium tungstate is obtained by digestion of tungsten ores, the economically important representatives of which are tungstates, in base. Illustrative is the extraction of sodium tungstate from wolframite:
Fe/MnWO4 + 2 NaOH + 2 H2O → Na2WO4?2H2O + Fe/Mn(OH)2
Sodium tungstate can also be produced by treating tungsten carbide with a mixture of sodium nitrate and sodium hydroxide in a fusion process which overcomes the high exothermicity of the reaction involved. | | Flammability and Explosibility | Not classified | | Safety Profile | Poison by ingestion, intravenous, intramuscular, and subcutaneous routes. Experimental reproductive effects. Mutation data reported. When heated to decomposition it emits toxic fumes of Na2O. See also TUNGSTEN COMPOUNDS. |
| | Sodium tungstate Preparation Products And Raw materials |
|