- Progabide
-

- $30.00 / 5mg
-
2024-11-19
- CAS:62666-20-0
- Min. Order:
- Purity: 99.7%
- Supply Ability: 10g
- Progabide USP/EP/BP
-

- $1.10 / 1g
-
2021-07-23
- CAS:62666-20-0
- Min. Order: 1g
- Purity: 99.9%
- Supply Ability: 100 Tons min
|
| | Progabide Basic information |
| Product Name: | Progabide | | Synonyms: | progabide;BUTANAMIDE, 4-[[(4-CHLOROPHENYL)(5-FLUORO-2-HYDROXYPHENYL)METHYLENE]AMINO]-;Halogabide;(E)-4-((4-chlorophenyl)(5-fluoro-2-hydroxyphenyl)methyleneamino)butanamide;4-[[(Z)-(4-Chlorophenyl)-(3-fluoro-6-oxo-1-cyclohexa-2,4-dienylidene)methyl]amino]butanamide;4-[[(4-Chlorophenyl)(5-fluoro-2hydroxyphenyl)methylene]amino]butanamide;C-abren(e);SL-76.002 | | CAS: | 62666-20-0 | | MF: | C17H16ClFN2O2 | | MW: | 334.77 | | EINECS: | 263-679-4 | | Product Categories: | Amines;Aromatics;Intermediates & Fine Chemicals;Pharmaceuticals | | Mol File: | 62666-20-0.mol | 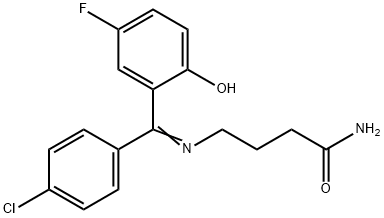 |
| | Progabide Chemical Properties |
| Melting point | 133-135°; mp 142.5° (Kaplan, J. Med. Chem.) | | Boiling point | 525.4±50.0 °C(Predicted) | | density | 1.2822 (estimate) | | storage temp. | 2-8°C | | solubility | Chloroform (Slightly, Sonicated), DMSO, Methanol (Slightly, Sonicated) | | form | Solid | | pka | pKa 3.41 ± 0.04(0.4% MeOH,t=37,Iundefined)(Approximate) | | color | Light Yellow to Yellow | | Water Solubility | 37.16mg/L(37 ºC) | | CAS DataBase Reference | 62666-20-0 |
| Toxicity | LD50 i.p. in mice: 900 mg/kg (Kaplan) |
| | Progabide Usage And Synthesis |
| Description | Progabide is a broad-spectrum anticonvulsant agent indicated for the treatment of
a wide variety of seizure disorders. Synthesized as a GABA-prodrug, anticonvulsant
activity appears due to the parent drug and its acid metabolite, as
well as to GABAmide and GABA liberated therefrom. | | Chemical Properties | Bright Yellow Solid | | Originator | Synthelabo (France) | | Uses | Progabide is a gamma-aminobutyric acid (GABA) antagonist with antiepileptic activity. | | Definition | ChEBI: Progabide is a diarylmethane. | | Brand name | Gabren (Synthelabo Pharmacie, France);Gabaphore;Sl 76 002;GABRENE. | | World Health Organization (WHO) | Progabide, an anticonvulsant, was introduced in France in 1985
for the treatment of epilepsy. Its use has occasionally been associated with
clinically evident signs of icteric hepatitis developing within the first six months of
treatment. These signs are generally reversible on withdrawal of the drug but
continuation of treatment has been associated with three reported fatalities (two of
which are doubtfully related to the drug). The manufacturer revised the data sheet
in March 1986 advising that use of progabide should be reserved for patients
unresponsive to other anticonvulsants. |
| | Progabide Preparation Products And Raw materials |
|