N,N'-OCTAMETHYLENEBIS(DICHLOROACETAMIDE) manufacturers
- WIN 18446
-
- $34.00 / 25mg
-
2024-11-19
- CAS:1477-57-2
- Min. Order:
- Purity: 99.69%
- Supply Ability: 10g
|
| | N,N'-OCTAMETHYLENEBIS(DICHLOROACETAMIDE) Basic information |
| Product Name: | N,N'-OCTAMETHYLENEBIS(DICHLOROACETAMIDE) | | Synonyms: | FERTILYSIN;N,N'-BIS(DICHLOROACETYL)-1,8-OCTAMETHYLENEDIAMINE;N,N'-OCTA-METHYLENE-BIS[2,2-DICHLOROACETAMIDE];N,N'-OCTAMETHYLENEBIS(DICHLOROACETAMIDE);bisdiamine;bis-diamine;n,n’,1,8-octanediylbis(2,2-dichloro-acetamid;n,n’-bis(dichloroacetyl)-1,8-diaminooctane | | CAS: | 1477-57-2 | | MF: | C12H20Cl4N2O2 | | MW: | 366.11 | | EINECS: | 216-033-0 | | Product Categories: | | | Mol File: | 1477-57-2.mol | 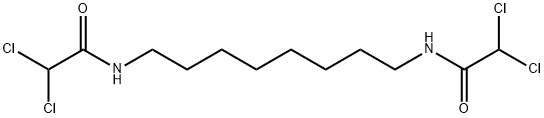 |
| | N,N'-OCTAMETHYLENEBIS(DICHLOROACETAMIDE) Chemical Properties |
| Melting point | 118-120 °C | | Boiling point | 530℃ | | density | 0.726 | | refractive index | 1.6200 (estimate) | | Fp | 275℃ | | storage temp. | Inert atmosphere,2-8°C | | solubility | Acetone (Very Slightly), DMSO (Slightly), Methanol (Sparingly, Heated, Sonicated | | pka | 12.28±0.46(Predicted) | | form | Solid | | color | White to Off-White | | CAS DataBase Reference | 1477-57-2 |
| Hazard Codes | Xn | | Risk Statements | 20/21/22-40 | | Safety Statements | 24/25-45-36 | | Toxicity | LD50 orally in mice: >16000 mg/kg (Coulston) |
| Provider | Language |
|
ACROS
| English |
| | N,N'-OCTAMETHYLENEBIS(DICHLOROACETAMIDE) Usage And Synthesis |
| Chemical Properties | white crystalline powder | | Uses | Win 18446 was shown to inhibit the biosynthesis of retinoic acid from retinol in neonatal and adult murine testis and in the embryonic murine gonad. | | Definition | ChEBI: A carboxamide that is 1,8-diaminooctane in which a hydrogen attached to each of the amino groups has been replaced by a dichloroacetyl group. Inhibitor of aldehyde dehydrogenase 1a2 (ALDH1a2). Inhibits the biosynthesis of retinoic acid from retinol in neon
tal and adult murine testis. It down-regulates sex related genes in zebrafish. | | Biological Activity | win 18446 is a bis-(dichloroacetyl)-diamine which inhibits aldehyde dehydrogenase 1a2 (aldh1a2), with ic50 value of 300 nm [1].aldh1a2, expressed almost exclusively in the testes, localizes to developing germ cells within the seminiferous tubules and strongly binds retinol, but does not recognize acetaldehyde as a substrate, suggesting that aldh1a2 functions in testicular retinoic acid biosynthesis [1].in whole testes or germ cells isolated from mice at 2 days postpartum, win 18,446 alone (1 μm) or in combination with retinol significantly reduced the expression of stra8, a marker for retinoic acid activity [2].in male rabbits, oral administration of win 18446 at 200 mg/kg for 16 weeks substantially reduced intratesticular concentrations of retinoic acid, significantly impaired spermatogenesis, and resulted in infertility. reduced concentrations of intratesticular retinoic acid became obvious after only 4 weeks of treatment and preceded the decrease in sperm counts and the loss of mature germ cells in tissue samples. sperm counts and fertility recovered after treatment was discontinued [1].[1]. amory j k, muller c h, shimshoni j a, et al. suppression of spermatogenesis by bisdichloroacetyldiamines is mediated by inhibition of testicular retinoic acid biosynthesis. journal of andrology, 2011, 32(1): 111-119.[2]. hogarth c a, evanoff r, snyder e, et al. suppression of stra8 expression in the mouse gonad by win 18,446. biology of reproduction, 2011, 84(5): 957-965. | | Safety Profile | Poison by intraperitoneal route.Human systemic effects by ingestion: nausea andvomiting. Human reproductive effects by ingestion:changes in spermatogenesis. An experimental teratogen.Other experimental reproductive effects. Human mutationdata reporte | | storage | Store at +4°C |
| | N,N'-OCTAMETHYLENEBIS(DICHLOROACETAMIDE) Preparation Products And Raw materials |
|