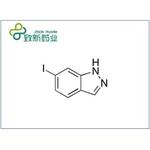
- 6-Iodo-1H-indazole
-

- $1.00 / 1kg
-
2024-11-15
- CAS:261953-36-0
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 200
- 6-IODO (1H)INDAZOLE
-

- $1.00 / 1KG
-
2024-11-13
- CAS:261953-36-0
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 10 mt
- 6-Iodo-1H-indazole
-
- $0.00 / 25kg
-
2024-09-23
- CAS:261953-36-0
- Min. Order: 1kg
- Purity: ≥98% by HPLC
- Supply Ability: 100kg/month
|
| | 6-Iodo-1H-indazole Chemical Properties |
| Melting point | 207.0 to 211.0 °C | | Boiling point | 358.2±15.0 °C(Predicted) | | density | 2.082±0.06 g/cm3(Predicted) | | storage temp. | under inert gas (nitrogen or Argon) at 2–8 °C | | solubility | DMSO (Slightly), Methanol (Slightly) | | pka | 12.96±0.40(Predicted) | | form | Liquid | | color | Clear colorless to light yellow | | InChI | InChI=1S/C7H5IN2/c8-6-2-1-5-4-9-10-7(5)3-6/h1-4H,(H,9,10) | | InChIKey | RSGAXJZKQDNFEP-UHFFFAOYSA-N | | SMILES | N1C2=C(C=CC(I)=C2)C=N1 |
| | 6-Iodo-1H-indazole Usage And Synthesis |
| Physical Form | Light yellow to Brown powder to crystal | | Uses | 6-Iodo-1H-indazole is an intermediate used to synthesize inhibitors of Chk1. | | Synthesis Reference(s) | [1] NOOLVI M N, PATEL H M. Small Molecule Tyrosine Kinase Inhibitors: The New Dawn for Cancer Therapy[J]. Letters in Drug Design & Discovery, 1900, 9(1): 84-125. DOI:10.2174/157018012798192892.
| | Synthesis | Revill P. and co-worker synthesized Axitinib by
iodination of 6-iodo-1H-indazole (100) to give 3,6-di iodo-
1H-indazole (101) ,which on further reaction with 2-
mercapto-N-methylbenzenesulfinamide gives 2-(3-iodo-1Hindazol-6-ylthio)-N-methylbenzamide (101), the same can be
obtained via 2-(1H-indazol-6-ylthion)-N-methylbenzamide
(102) starting from 6-iodo-1H-indazole (100) through
alternative route. N-protection of 2-(3-iodo-1H-indazol-6-
ylthio)-N-methylbenzamide (101) by Boc2, DMAP or DHP,
TsOH gives respective N-protected intermediates 103, 104.
Further treatment of 103, 104 with 2-vinyl pyridine give rise
to N-protected-2-(1-methyl-3-(2-(pyridine-2-yl) vinyl)-1Hindazole-6-ylthio) benzothioamide 105 and 106. Deprotection of 105 and 106 gives destination compound
axitinib (107), the same can be obtained without protection
from 2-(3-iodo-1H-indazol-6-ylthio)-N-methylbenzamide
(101) by simple reaction with 2-vinyl pyridine[1].
 |
| | 6-Iodo-1H-indazole Preparation Products And Raw materials |
|