|
|
| | GGTI 298 trifluoroacetate salt hydrate Basic information |
| Product Name: | GGTI 298 trifluoroacetate salt hydrate | | Synonyms: | GGTI 298 trifluoroacetate salt hydrate;N-[[4-(2-(R)-Amino-3-mercaptopropyl)amino]-2-naphthylbenzoyl]leucine methyl ester trifluoroacetate salt hydrate;GGTI-298 TFA;GGTI298 Trifluoroacetate;WALKWJPZELDSKT-UFABNHQSSA-N;GGTI 298 TFA salt;GGTI 298 TFA SALT;GGTI 298 (TRIFLUOROACETATE SALT);(S)-Methyl 2-(4-(((R)-2-amino-3-mercaptopropyl)amino)-2-(naphthalen-1-yl)benzamido)-4-methylpentanoate 2,2,2-trifluoroacetate | | CAS: | 1217457-86-7 | | MF: | C29H34F3N3O5S | | MW: | 593.66 | | EINECS: | | | Product Categories: | | | Mol File: | 1217457-86-7.mol | 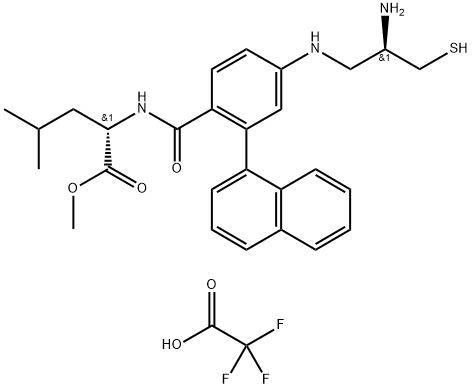 |
| | GGTI 298 trifluoroacetate salt hydrate Chemical Properties |
| Melting point | 99.5-100°C | | storage temp. | -20°C | | solubility | DMSO: >20mg/mL | | form | film &_& lyophilized | | color | colorless |
| Hazard Codes | Xi | | Risk Statements | 36/37/38 | | Safety Statements | 26-36 | | WGK Germany | 3 |
| | GGTI 298 trifluoroacetate salt hydrate Usage And Synthesis |
| Uses | GGTI 298 trifluoroacetate salt hydrate has been used as a geranylgeranyltransferase I (GGTase I) inhibitor:
- to study the anticancer effects of statins along with GGTI 298
- to study its combinatorial effects with FTI-277 on statin-mediated activation of extracellular signal-regulated kinase 5 (ERK5) in the human endothelium
- to evaluate the effect of protein geranylgeranylation (GG) inhibition on the breast cancer stem cell (CSC) population
| | Biochem/physiol Actions | GGTI 298 is a cell-permeable, prodrug form of the geranylgeranyltransferase I (GGTase I) inhibitor GGTI-297. It inhibits the processing of Rap 1A without effecting the processing of H-Ras. | | storage | Store at -20°C |
| | GGTI 298 trifluoroacetate salt hydrate Preparation Products And Raw materials |
|