- Methoxychlor
-

- $30.00 / 10mg
-
2024-11-19
- CAS:72-43-5
- Min. Order:
- Purity: ≥95%
- Supply Ability: 10g
|
| | METHOXYCHLOR Basic information |
| Product Name: | METHOXYCHLOR | | Synonyms: | 1,1'-(2,2,2-Trichloroethylidene)bis(4-methoxybenzene);1,1,1-Trichlor-2,2-bis(4-methoxy-phenyl)-aethan;1,1,1-Trichloro-2,2-bis(p-anisyl)ethane;1,1,1-trichloro-2,2-bis(p-methoxyphenol)ethanol;1,1,1-trichloro-2,2-bis(p-methoxyphenyl)-ethan;1,1,1-Trichloro-2,2-di(4-methoxyphenyl)ethane;1,1,1-Trichloro-2,2-bis-(p-methoxyphenyl)ethane, 2,2-Bis(4-methoxyphenyl)-1,1,1-trichloroethane, DMDT;Dairy Cattle | | CAS: | 72-43-5 | | MF: | C16H15Cl3O2 | | MW: | 345.65 | | EINECS: | 200-779-9 | | Product Categories: | Alpha sort;H-MAlphabetic;META - METHPesticides;Oeko-Tex Standard 100;OrganochlorinesMethod Specific;Pesticides&Metabolites;Insecticides;M;Pesticides;Organochlorines;INSECTICIDE;Organics | | Mol File: | 72-43-5.mol | 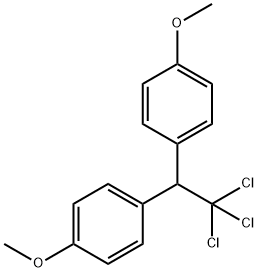 |
| | METHOXYCHLOR Chemical Properties |
| Melting point | 86-88 °C(lit.) | | Boiling point | 346 °C | | density | 1.41 g/cm3 (25℃) | | vapor pressure | Very low | | Fp | 11 °C | | storage temp. | APPROX 4°C | | solubility | Soluble in ethanol (Windholz et al., 1983), chloroform (440 g/kg), xylene (440 g/kg), and
methanol (50 g/kg) (Worthing and Hance, 1991) | | Water Solubility | 0.1 mg l-1 (25 °C) | | form | crystalline | | color | yellow | | Merck | 14,5990 | | BRN | 2057367 | | Exposure limits | NIOSH REL: IDLH 5,000 mg/m3; OSHA PEL: TWA 15 mg/m3;
ACGIH TLV: TWA 10 mg/m3. | | Stability: | Stable, but light sensitive. Combustible at high temperature. Corrodes aluminium and iron slowly. | | CAS DataBase Reference | 72-43-5(CAS DataBase Reference) | | IARC | 3 (Vol. 20, Sup 7) 1987 | | EPA Substance Registry System | Methoxychlor (72-43-5) |
| | METHOXYCHLOR Usage And Synthesis |
| Description | Methoxychlor is a structural analogue of DDT but is not
as persistent in the environment as DDT. | | Chemical Properties | Methoxychlor is a colourless or pale yellow, crystalline synthetic organochlorine insecticide with mild fruity odour. It is available in the form of powder or crystals. Methoxychlor is moderately soluble in water and is soluble in a variety of organic solvents. | | Physical properties | White to gray, or pale yellowish-orange crystals or powder. Nonflammable but may be combustible
if dissolved in a flammable organic solvent or petroleum distillate for application. Pungent to
mild, fruity odor. Odor threshold concentration in water is 4.7 mg/kg (quoted, Keith and Walters,
1992). | | Uses | Insecticide used to control mosquito larvae, house flies and other insect pests in
field crops, fruits and vegetables; to control ecto-parasites on cattle, sheep and goats;
recommended for use in dairy barns. | | Uses | Insecticide effective against mosquito larvae
and house flies; recommended for use in dairy
barns. | | Uses | Methoxychlor is effective against flies, mosquitos, cockroaches,
and a wide variety of other insects. This insecticide is used on
agricultural crops and livestock, and in animal feed, barns, and
grain storage bins. Some pesticide products that contain
methoxychlor are used for controlling insects in gardens or on pets. | | Definition | ChEBI: Methoxychlor is an organochlorine insecticide. It is functionally related to a 1,1,1-trichloro-2,2-diphenylethane. | | General Description | METHOXYCHLOR is a white crystalline solid which is often dissolved in a liquid carrier such as diesel oil. METHOXYCHLOR can cause illness by inhalation, skin absorption and/or ingestion. The primary hazard is the threat to the environment. Immediate steps should be taken to limit its spread to the environment. If dissolved in a liquid carrier, METHOXYCHLOR can easily penetrate the soil and contaminate groundwater and nearby streams. METHOXYCHLOR is used as a pesticide. | | Air & Water Reactions | Insoluble in water. | | Reactivity Profile | METHOXYCHLOR turns pink or tan on exposure to light. METHOXYCHLOR is incompatible with alkaline materials, especially in the presence of catalytically-active metals. METHOXYCHLOR is slightly corrosive to iron and aluminum. METHOXYCHLOR is decomposed by refluxing with sodium in isopropyl alcohol. METHOXYCHLOR is also incompatible with strong oxidizers. METHOXYCHLOR will attack some forms of plastics, rubber and coatings. . | | Hazard | Toxic material. Liver damage and central
nervous system impairment. Questionable carcinogen. | | Health Hazard | Low toxicity; acute and chronic effects aremuch less serious than those of the structurally similar DDT; skin absorption low;may cause kidney damage on chronic exposure; oral LD50 value (rats): 6000 mg/kg(ACGIH 1986); no evidence of carcinogenicity to animals or humans; exposure limit:TLV-TWA 10 mg/m3 (ACGIH, MSHA, andOSHA); RCRA Waste Number U247
Methoxychlor is a reproductive toxicant.Exposure of young adult male rats to methoxychlor reduced their serum testosterone levels (Murono et al. 2006). It metabolized in theliver into 2,2-bis(p-hydroxyphenyl)-1,1,1-trichloroethane and both methoxychlor and itsmetabolite were found to exhibit weak estrogenic and antiandrogenic activities. Flynnet al. (2005) have reported that long-termexposure to methoxychlor in diet altered thesexually dimorphic behavior in young ratsof both sexes. The study indicated that con�sumption of sodium solution increased in ratsfrom such exposure. The toxicity of methoxy�chlor, particularly in the central nervous sys�tem, may be attributed to its inhibition of brainmitochondrial respiration which probably iscaused by an increased production of reactiveoxygen species (Schuh et al. 2005). | | Health Hazard | Toxicity is relatively low. Inhalation or ingestion causes generalized depression. | | Fire Hazard | Special Hazards of Combustion Products: Irritating and toxic hydrogen chloride gas may be formed in fire. | | Agricultural Uses | Insecticide: Not approved for use in EU countries
. Not registered for use in the U.S. There are 33 global suppliers
.
The U.S. EPA lists 826 active and/or canceled products
containing methoxychlor. Methoxychlor was introduced
as an insecticide in 1945. It is a close relative of DDT
and has been increasing in use since the ban on DDT in
1972 because of its very low mammalian toxicity for home
and garden, on domestic animals for fly control, for elm
bark-beetle vectors of Dutch elm disease, and for blackfly larvae in streams. Methoxychlor is registered for about
87 crops such as alfalfa; nearly all fruits and vegetables,
corn, wheat, rice, and other grains; beef and dairy cattle;
and swine, goats and sheep, and for agricultural premises
and outdoor fogging. It is available in wettable and dust
powders, emulsifiable concentrates, granules, and as an
aerosol. It is combined in formulations with malathion,
parathion, piperonyl butoxide, and pyrethrins. | | Trade name | CHEMFORM®; HIGALMETOX®;
MARLATE®; METOX®; MOXIE®; PRENTOX® | | Safety Profile | Suspected carcinogen
with experimental carcinogenic,
tumorigenic, and teratogenic data.
Moderately toxic by intraperitoneal and
skin contact routes. Human systemic
effects by skin contact: somnolence.
Experimental reproductive effects. Mutation
data reported. When heated to
decomposition it emits highly toxic fumes of
Cl-. See also DDT and
CHLOROPHENOLS. | | Potential Exposure | Agricultural Chemical; Tumorigen, Mutagen; Reproductive Effector; Human Data. Methoxychlor was introduced as an 1734 Methoxychlor insecticide in 1945. It is a close relative of DDT and has been used as an insecticide of very low mammalian toxicity for home and garden, on domestic animals for fly control, for elm bark-beetle vectors of Dutch elm disease, and for blackfly larvae in streams. Methoxychlor is registered for about 87crops, alfalfa, nearly all fruits and vegetables, corn, wheat, rice, and other grains, beef and dairy cattle, and swine, goats and sheep, and for agricultural premises and outdoor fogging. Thus, those engaged in manufacture, formulation, and application of the material as well as people in application areas may be exposed. | | First aid | If this chemical gets into the eyes, remove any contact lenses at once and irrigate immediately for at least 15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts the skin, remove contaminated clothing and wash immediately with soap and water. Seek medical attention immediately. If this chemical has been inhaled, remove from exposure, begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR if heart action has stopped. Transfer promptly to a medical facility. When this chemical has been swallowed, get medical attention. Give large quantities of water and induce vomiting. Do not make an unconscious person vomit. | | Carcinogenicity | Female mice fed up to 2000mg/kg and
males given 3500 mg/kg in the diet for 78
weeks showed no statistically significant
increase in the incidence of benign and malignant
tumors that could be attributed to
methoxychlor.10 Chronic feeding studies in
rats, at 850 and 1400mg/kg for males and
females, respectively, also showed no significant
carcinogenic responses, although high
tumor rates in controls may have masked
detection.10 Based on NCI results and several
earlier animal studies, the IARC has determined
that there is insufficient evidence that
methoxychlor is carcinogenic in experimental
animals and that it is not classifiable as to its
carcinogenicity to humans. | | Environmental Fate | Biological. Degradation by the microorganism Aerobacter aerogenes under aerobic or
anaerobic conditions yielded 1,1-dichloro-2,2-bis(p-methoxyphenyl)ethylene and 1,1-
dichloro-2,2-bis(p-methoxyphenyl)ethane (Mendel and Walton, 1966; Kobayashi and Rittman,
1982). Methoxychlor degrades at a faster rate in flooded/anaerobic soils than in
nonflooded and aerobic soils (Fogel et al., 1982; Golovleva et al., 1984). In anaerobic soil,
90% of the applied dosage was lost after 3 months. In aerobic soil, only 0.3% was lost
as carbon dioxide after 410 days (Fogel et al., 1982). In a modified river die-away test,
methoxychlor underwent aerobic biodegradation at a rate of 0.0024/hour which corresponds
to a half-life of 12 days (Cripe et al., 1987).
In a model aquatic ecosystem, methoxychlor degraded to ethanol, dihydroxyethane,
dihydroxyethylene and unidentified polar metabolites (Metcalf et al., 1971). Kapoor et al.
(1970) also studied the biodegradation of methoxychlor in a model ecosystem contain
From the first-order biotic and abiotic rate constants of methoxychlor in estuarine
water and sediment/water systems, the estimated biodegradation half-lives were 208–8,837
and 12–45 days, respectively (Walker et al., 1988).
Paris and Lewis (1976) reported that the microorganism Aspergillis sp. accumulated
methoxychlor slowly, requiring 16 hours to reach equilibrium. Some microorganisms, such
as Flavobacterium harrisonii, Bacillus subtilis and Chlorella pyrenoidoda accumulated
methoxychlor and reached equilibrium in only 30 minutes.
Groundwater. According to the U.S. EPA (1986) methoxychor has a high potential to
leach to groundwater. | | Metabolic pathway | Upon UV irradiation with methyl oleate, methoxychlor
is extensively added to the carbon ? carbon double
bond of methyl oleate via radical mechanisms.
Besides chlorinated stearic acids, several addition
products are formed, offering new possibilities to
produce bound residues in plants. The incubation of
methoxychlor with liver microsomes from untreated
and phenobarbital-treated rats and donors, in the
presence of NADPH, yields three phenolic
metabolites: monodemethylated and didemethylated
methoxychlor and its hydroxylated (trihydroxy)
methoxychlor. The metabolic route of methoxychlor by
monooxygenases involves sequential demethylations
to the dihydroxy derivative and a subsequent ring
hydroxylation. | | Solubility in water | Soluble in ethanol (Windholz et al., 1983), chloroform (440 g/kg), xylene (440 g/kg), and
methanol (50 g/kg) (Worthing and Hance, 1991) | | storage | Color Code—Blue: Health Hazard/Poison: Store in a secure poison location. Protect containers from damage. Store in cool, dry area away from fire hazard and out of direct sunlight. | | Shipping | This material may be classed under Organochlorine Pesticides, solid, toxic, n.o.s. This compound requires a shipping label of “POISONOUS/TOXIC MATERIALS.” It falls in Hazard Class 6.1 and Packing Group III. | | Purification Methods | Free the insecticide from 1,1-bis(p-chlorophenyl)-2,2,2-trichloroethane by crystallising from EtOH. It is dimorphic and also crystallises from Et2O/EtOH (m 92o). [Fritsch & Feldmann Justus Liebigs Ann Chem 306 77 1899, Smith et al. Aust J Chem 29 743 1976, Beilstein 6 H 1007.] | | Toxicity evaluation | Acute oral LD50 for rats: 6,000 mg/kg | | Degradation | Methoxychlor is stable to oxidising agents and to ultraviolet light but
it becomes pink or tan-coloured on irradiation. It reacts with alkalis,
especially in the presence of catalytic metals, with the loss of hydrogen
chloride to give 1,l-dichloro-2,2-bis(4-methoxyphenyl)ethylene(2).
The major product of photolysis of methoxychlor in air-saturated water,
irradiated at wavelengths >280 nm, was 2 while 1,1-dichloro-2,2-bis(4-
methoxyphenyl)ethane (3) was formed along with 2 in degassed wateracetonitrile
solutions. Subsequently, 2 was photolysed to benzaldehyde
(Zepp et al., 1976). Products of photolysis in aqueous alcoholic solutions
were 4,4’-dimethoxybenzophenone (4), 4-methoxybenzoic acid, and 4-
methoxyphenol(5) (Wolfe et al., 1976). When methoxychlor was irradiated
in milk by ultraviolet light (carbon arc, 220-330 nm) compounds 4 and 5, 4-methylanisole (6), 1,1,4,4-tetrakis(4-methoxyphenyl)-2,3-dichloro-2-
butene (7) and 1,1,4,4-tetrakis(4-methoxyphenyl)-1,2,3-butatriene(8) were
identified as products (Li and Bradley, 1969) (Scheme 1).
In water, chemical decomposition was slow and at 27 °C the DT50 at pH
5 to 9 was 100 days. Major products of hydrolysis are anisoin (9), anisil
(10) and 2 (Wolfe et al., 1977) (see Scheme 2). | | Incompatibilities | Oxidizers. |
| | METHOXYCHLOR Preparation Products And Raw materials |
|