|
|
| | 5-Bromo-2-pyridinecarbonitrile Basic information |
| | 5-Bromo-2-pyridinecarbonitrile Chemical Properties |
| Melting point | 128-132 °C (lit.) | | Boiling point | 100-110 °C/3 mmHg (lit.) | | density | 1.72±0.1 g/cm3(Predicted) | | storage temp. | Inert atmosphere,Room Temperature | | solubility | Soluble in dichloromethane, ether, ethyl acetate and methanol | | form | powder to crystal | | pka | -2.68±0.10(Predicted) | | color | White to Light yellow to Light orange | | BRN | 5498624 | | InChI | InChI=1S/C6H3BrN2/c7-5-1-2-6(3-8)9-4-5/h1-2,4H | | InChIKey | DMSHUVBQFSNBBL-UHFFFAOYSA-N | | SMILES | C1(C#N)=NC=C(Br)C=C1 | | CAS DataBase Reference | 97483-77-7(CAS DataBase Reference) |
| | 5-Bromo-2-pyridinecarbonitrile Usage And Synthesis |
| Chemical Properties | Light yellow Cryst | | Uses | 5-Bromo-2-pyridinecarbonitrile can be used in the synthesis of aza-terphenyl diamidine analogs, which exhibits potent antiprotozoal activity. It can also be used in the synthesis of pyridine-diketopyrrolopyrrole(PyDPP), a building block for preparing low band-gap copolymers for use as electron donor in polymer solar cells. | | Chemical Properties | The intramolecular hyperconjugative interactions of the p to p* transitions from (C1—C2, C3—C4. C5—N6) p bonds in pyridine ring lead to strong delocalization. In particular, there is a little deviation in the energetic contributions from p (C1—C2 and C3—C4) bonds to p* [(C3—C4, C5—N6) and (C1—C2, C5—N6)] antibond transitions of (22.96, 17.04 kJ mol1 ) and (18.04, 24.81 kJ mol1 ), respectively, compared to other contributions in the ring. As well as the hyperconjugative interactions of the s / s* transitions occur from various bonds in our molecule; particularly, σ (C1—C11) and (C11—N12) having the bigger energetic contribution of their antibonding σ* (C11—N12) and (C1—C11) interactions at 8.28 and 8.68 kJ mol1 , respectively[1]. The most interaction energy, related to the resonance in the molecule, electron donating from the LP(2) N12 to the antibonding σ* (C1—C11) leads to moderate stabilization energy of 11.95 kJ mol1 is shown below.
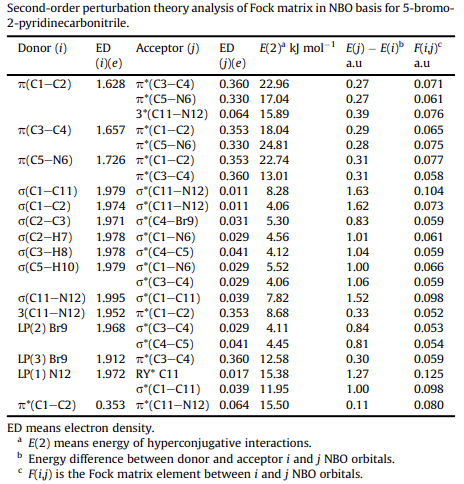 | | References | [1] Kandasamy, M. , and G. Velraj . "Ab initio/DFT electronic structure calculations, spectroscopic studies of 5-bromo-2-pyridinecarbonitrile – A comparative study." Solid State Sciences 14.8(2012):1071-1079. |
| | 5-Bromo-2-pyridinecarbonitrile Preparation Products And Raw materials |
|