|
|
| | 4-bromoisoindolin-1-one Basic information |
| Product Name: | 4-bromoisoindolin-1-one | | Synonyms: | 4-bromoisoindolin-1-one;4-BroMoisoindoline-1-one;1H-Isoindol-1-one,4-broMo-2 3-dihydro-;4-broMo-2,3-dihydro-1H-isoindol-1-one;4-Bromoisoindolin-1-one 97%;Ethanone,1-[3,6-dihydroxy-2-methoxy-7-(phenylmethoxy)phenyl]-;4-Bromo-1-isoindolinone;4-Bromoisoindolin-1-one?New | | CAS: | 337536-15-9 | | MF: | C8H6BrNO | | MW: | 212.04 | | EINECS: | | | Product Categories: | | | Mol File: | 337536-15-9.mol | 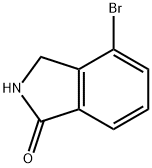 |
| | 4-bromoisoindolin-1-one Chemical Properties |
| Boiling point | 448.9±45.0 °C(Predicted) | | density | 1.666 | | storage temp. | Sealed in dry,Room Temperature | | pka | 13.69±0.20(Predicted) | | form | solid | | color | Off-white to light yellow |
| | 4-bromoisoindolin-1-one Usage And Synthesis |
| Uses | 4-bromoisoindolin-1-one can be used as organic synthesis intermediate and pharmaceutical intermediate, mainly used in laboratory research and development process and chemical production process. | | Synthesis | 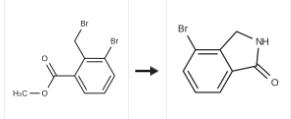
To a solution of the 3-bromo-2-bromomethyl-benzoic acid methyl ester (2.74 g, 8.88 [MMOL)] in tetrahydrofuran (70 [ML)] at [0??C] was added 30 percent aq. ammonia (10 ml) and the mixture stirred at room temperature under nitrogen for 18 hours. The solvent was removed by evaporation under reduced pressure. The white residue was partitioned between ethyl acetate (50 mi) and 2M citric acid (50 [ML).] The ethyl acetate was dried magnesium sulfate, filtered and solvent removed by evaporation under reduced pressure. The orange oil was dissolved in minimum [DICHLOROMETHANE] and purified by flash chromatography on silica gel eluting with a solvent gradient of [DICHOROMETHANE/METHANOL] (9: 1) to give the title compound (1.5 g, 80 percent) as a white solid. |
| | 4-bromoisoindolin-1-one Preparation Products And Raw materials |
|