- D-Calcium Pantothenate
-

- $0.00 / 1Kg/Bag
-
2025-03-29
- CAS:137-08-6
- Min. Order: 1KG
- Purity: 98.0%-102.0%;USP
- Supply Ability: 10 TONS
Related articles - What is D-Calcium pantothenate?
- D-calcium pantothenate known as pantothenic acid, is a typical nutritional fortification substance of the B-complex vitamins t....
- Feb 25,2020
|
| Product Name: | Calcium D-Pantothenate | | Synonyms: | (+)-PANTOTHENIC ACID CALCIUM SALT;(R)-(+)-N-(2,4-DIHYDROXY-3,3-DIMETHYL-1-OXOBUTYL)-BETA-ALANINE HEMICALCIUM SALT;(theta)-;beta-alanine,n-(2,4-dihydroxy-3,3-dimethyl-1-oxobutyl)-,calciumsalt(2:1),;N-(2,4-dihydroxy-3,3-dimethyl-1-oxobutyl)-,calciumsalt(2:1),(R)-.beta.-Alanine;N-(2,4-Dihydroxy-3,3-dimethyl-1-oxobutyl)-alaninecalciumsalt;n-(2,4-dihydroxy-3,3-dimethylbutyryl)-beta-alaninecalcium;panthoject | | CAS: | 137-08-6 | | MF: | C9H17NO5.1/2Ca | | MW: | 476.53 | | EINECS: | 205-278-9 | | Product Categories: | Aliphatics;Chiral Reagents;Intermediates & Fine Chemicals;Pharmaceuticals;Pyridines ,Halogenated Heterocycles;Food additives;Isolabel;Biochemistry;Ca (Calcium) Compounds;Classes of Metal Compounds;Typical Metal Compounds;Vitamins;Nutritional Supplements;Nutritional fortification substances | | Mol File: | 137-08-6.mol | 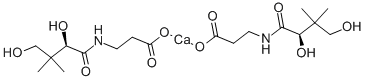 |
| | Calcium D-Pantothenate Chemical Properties |
| Melting point | 190 °C | | alpha | 26.5 º (c=5, in water) | | refractive index | 27 ° (C=5, H2O) | | Fp | 145 °C | | storage temp. | 2-8°C | | solubility | H2O: 50 mg/mL at 25 °C, clear, nearly colorless | | form | Powder | | color | White or almost white | | Odor | Odorless | | PH | 6.8-7.2 (25℃, 50mg/mL in H2O) | | optical activity | [α]20/D +27±2°, c = 5% in H2O | | biological source | synthetic (organic) | | Water Solubility | Soluble in water. | | Sensitive | Hygroscopic | | Merck | 14,7015 | | BRN | 3769272 | | Stability: | Stable, but may be moisture or air sensitive. Incompatible with strong acids, strong bases. | | InChIKey | FAPWYRCQGJNNSJ-UBKPKTQASA-L | | LogP | -0.849 (est) | | CAS DataBase Reference | 137-08-6(CAS DataBase Reference) | | EPA Substance Registry System | Calcium pantothenate (137-08-6) |
| | Calcium D-Pantothenate Usage And Synthesis |
| Nutritional supplement | D-Calcium pantothenate as components of coenzyme A regulates the metabolism of protein, saccharide, and fat, and prevents diseases, which is indispensable substance for growth and development of domesticated creatures and fishes, for fatty synthesis and decomposition. The lack of D-calcium pantothenate would result in the slow growth of poultry and the malfunction of reproduction mechanisms. Therefore, D-calcium pantothenate as a growth factor is used in feed additives. In addition, D-calcium pantothenate is also as food enrichment widely used in food industries, sun as breakfast cereals, beverages, dietetic, and baby foods.
D-Calcium pantothenate has the function of making antibodies and it plays an important role in the fight against pressure to maintain hair, skin and blood health, and also contributes to improving the deficiency and neuritis. Thus, it has broad medical value and has been applied in pharmaceutical industries that single-dose is used for pantothenic acid deficiency, complex of vitamins B and multivitamins are used for vitamin supplement, and other compounds with different components are widely used for gastrointestinal diseases, respiratory diseases, skin diseases, mental inactivity, neurasthenia, and so on.
| | Production method | Take isobutyraldehyde as raw material; carry out hydroxymethylation, addition reaction, hydrolysis, acidification, lactonization and acylation to derive the final product.
Formaldehyde and anhydrous potassium carbonate are sent into the reaction pot. At 14-20 °C, it is added dropwise of isobutyraldehyde. After the completion of the addition, incubate for stirring reaction for 3h, and then stand at 14-18 °C for 0.5 h to obtain the upper oil-2, 2-dimethyl-3-hydroxypropionaldehyde.
2, 2-dimethyl-3-hydroxypropanal is dissolved in 4 times the water while the sodium cyanide is dissolved in 6 times the water and calcium chloride dissolved in 2 times the water. The above solutions are successively added into the reaction pot. 50% sulfuric acid solution was added under stirring at 60-65 ° C for 6h; then being heated to 80-85 °C for 3h and subject to vacuum concentration to being thick. 95% ethanol was added to precipitate the inorganic salt and filtrate. After the recovery of ethanol through distillation under reduced pressure, collect the fractions of 130-145C (1.33-2.39 Kpa), namely ?-butyrolactone.
Then, β-aminopropionic acid, 5/6 methanol and lime were successively added to the reactor. Raise the temperature to 40 °C, and the reaction was stirred for 2 hours. The reaction mixture was allowed to stand for while before the supernatant was filtered by filtration. The solid residue in the pot was washed with 1/6 methanol and then filtered. The filtrate is placed in another reaction pot, being added of γ-butyrolactone for stirring and dissolving in room temperature for 40 h reaction to generate calcium pantothenate. Stirring with water and cooling to-5-0 °C, and add seed crystal for stirring of 24 hours, filter to obtain the dextro calcium pantothenate.
2/3 the amount of calcium pantothenate and calcium p-pantothenate are thrown into the reaction pot; add methanol and water, heat to 40 °C for stirring dissolving and filter upon being hot. The filtrate is cooled to 15 °C, added of a small amount of calcium L-pantothenate and crystal for 2h. When the specific rotation is up to +6 °-+8 °, separate the crystal, and wash with a small amount of methanol to obtain the L-calcium p-pantothenate (still used for resolution). The remaining 1/3 racemic calcium pantothenate was dissolved in a filtrate of 35-40 ° C, filtered and cooled to 15 ° C. A small amount of calcium p-pantothenate was added and the crystals were incubated for 2 hours. Crystals were separated when the specific rotation was-0.8 °--0.6 °, washed with a small amount of methanol and dried in vacuo to give calcium dextrate.
It is derived through the heating and condensation between calcium β-alanine and α-hydroxy β, β-dimethyl-γ-butyl ester. | | Uses | 1. It can be applied to biochemical studies; as the nutrient composition of tissue culture medium. It is clinically used for the treatment of vitamin B deficiency, peripheral neuritis and postoperative colic.
2. It can be used as food fortifier, also used as infant food with the usage amount of 15~28 mg/kg; it is 2~4mg/kg in the drink.
3. This product is a vitamin drugs, being an integral part of coenzyme A. In the mixture of calcium pantothenate, only the right-hand body has vitamin activity, participating into the in vivo metabolism of protein, fat and carbohydrate. It can be used for the treatment of vitamin B deficiency and peripheral neuritis, and postoperative colic. Its combined treatment with vitamin C can be used for the treatment of disseminated lupus erythematosus. The lack of calcium pantothenate in human body has the following symptoms: (1) growth arrest, weight loss and sudden death. (2) Skin and hair disorders. (3) Neurological disorders. (4) Digestive disorders, liver dysfunction. (5) Affect the antibody formation. (6) Kidney dysfunction. Every day the body demands 5 mg of calcium pantothenate (calculated based on pantothenic acid). Calcium pantothenate, as a nutritional supplement, can be used for the food processing. In addition to special nutritional food, the usage amount should be below 1% (calculated on calcium) (Japan). Upon the strengthening of the milk powder, the usage amount should be 10 mg/100g. Addition of 0.02% into the Shochu and whiskey can further enhance the flavor. Addition of 0.02% into the honey can prevent the winter crystallization. It can be used for buffering the bitterness of caffeine and saccharin.
4. It can be used as feed additives, food additives, being in line with Pharmacopoeia USP28/BP2003
5. It can be used as nutritional supplements, being able to enhance the flavor of shochu whiskey to prevent the crystallization of honey in winter.
6. It is the precursor product for the biosynthesis of coenzyme A. Because of the easy-deliquescence of pantothenic acid and other unstable properties, it is used of calcium salt as the substitute. | | Toxicity | LD50>10 g/kg (rat, oral);
GRAS (FDA, § 182.5212, §184.1212, 2000); | | Usage limit | GMP as the limit (FDA &184.1212, 2000);
1% in general food (calculated based on calcium excluding special nutritional supplements; Japan, 1993); | | Chemical properties | It appears as white crystalline (methanol), being hygroscopic; it is stable to the light and air with its aqueous solution being weakly alkaline. Its Mp is 195-196 °C (decomposition); Specific rotation [α] 26D + 28.2 ° (5%, water); It is soluble in water and glycerol, being slightly soluble in acetone and ethanol; | | Hazards & Safety Information | Category: Toxic substances
Toxicity classification: Low toxicity
Acute toxicity: Oral-rat LD50: 10000 mg/kg; oral-mouse LD50: 10000 mg/kg
Flammability and Hazardous characteristics: Thermal decomposition releases toxic nitrogen oxides
Storage and transport characteristics Treasury: low temperature, ventilated and dry
Fire extinguishing agent: water, carbon dioxide, dry powder, foam | | Chemical Properties | Calcium D-Pantothenate is white crystalline powder | | Originator | Calcium D-Pantothenate,Arocor Holdings Inc. | | History | Pantothenic acid (PA), also known as vitamin B5, is essential to all forms of life. Its name is derived from the Greek word pantos that means “everywhere”, which is appropriate for this widely, distributed vitamin. | | Uses | Calcium Pantothenate is a nutrient and dietary supplement which is the calcium chloride double salt of . It is a white powder of bitter taste and has a solubility of 1 g in 3 ml of water. It is used in special dietary foods. | | Uses | calcium pantothenate is used as an emollient and to enrich creams and lotions in hair care preparations. This is the calcium salt of pantothenic acid found in liver, rice, bran, and molasses. It is also found in large amounts in royal jelly. | | Uses | D-(+)-Pantothenic acid calcium salt is a member of the B complex vitamins; essential vitamin for the biosynthesis of coenzyme A in mammalian cells. Occurs ubiquitously in all animal and plant tissue. The richest common source is liver, but jelly of the queen bee contains 6 times as much as liver. Rice bran and molasses are other good sources. | | Definition | Calcium pantothenate, also known as calcium D-pantothenate or calcium pantothenic acid, is a naturally occurring form of pantothenic acid, an essential vitamin found in various foods. Calcium pantothenate is generally recognized as safe (GRAS) in the United States and in Europe. The US FDA requires infant formulas have at least 300 micrograms (mcg) per 100 calories of prepared formula. | | Definition | ChEBI: Calcium pantothenate is a polymer. | | Manufacturing Process | A mixture of 288 g (4 mols) of isobutyraldehyde, 288 g of methanol was
cooled to 10°C and 170 g (2 mols) of 36.6% formalin containing 8.5 g (3%
based on isobutyraldehyde) of sodium hydroxide was added dropwise over a
55 minute period to produce alpha,alpha-dimethyl-beta-hydroxy-propionaldehyde. The mixture was stirred for an additional 2 hours at 10-15°C
and then contacted with acetic acid to neutralize the catalyst. The excess
isobutyraldehyde and methanol were stripped off at a kettle temperature of
50°C at 25 mm. To the residual α,α-dimethyl-beta-hydroxypropionaldehyde a
mixture of 260 ml of methanol and 2 g (0.75%) sodium cyanide was added
and the solution cooled to 10°C before adding 59.4 g (2.2 mols) of hydrogen
cyanide dropwise over a 35 minute period to produce α,γ-dihydroxy-β,β-
dimethylbutyronitrile. The mixture was stirred at 10°C for one hour period and
then contacted with acetic acid to neutralize the catalyst before stripping off
the excess methanol to a kettle temperature of 45°C at 18 mm. The crude
cyanohydrin was then hydrolysed by heating with 4 mols of concentrated
hydrochloric acid at 80°C for 2 hours, then diluting with an equal volume of
water and heating at 100°C for an additional 8 hours. The aqueous mixture
was extracted continuously with ethylene dichloride. The solvent was removed, and pantolactone (B. P. 131°C/19 mm, M.P. 61-77°C, 96.5% purity
by saponification) was obtained by distillation in 71.5% yield based on
formaldehyde and 55% efficiency based on isobutyraldehyde.
26 grams of racemic pantolactone (0.2 mol) and 1.1 grams of sodium
methoxide (0.02 mol) contained in 30 ml of methanol, were added to 78.8
grams of 1-brucine (0.2 mol) contained in 156 ml of methanol. The resulting
mixture was refluxed for 1.5 hours and allowed to stand at room temperature
overnight. After centrifuging, washing with methanol and drying, 65.4 grams
of D-(-)-pantolactone 1-brucine (62% of theory based upon all of the racemic
pantolactone) melting at 203° to 206°C were obtained. Upon chilling the
mother liquor, 13.46 grams of additional complex melting at 175° to 177°C
were obtained.
D-(-)-Pantolactone was obtained from the complex in the following manner.
The 65.4 grams of complex obtained above were treated with 65 ml of
chloroform and 5.35 grams of sodium hydroxide contained in 35 ml of water
for one hour at room temperature. The aqueous layer was extracted 6 times
with 20 ml portions of chloroform in order to remove the brucine. The sodium
pantoate contained in the aqueous layer was relactonized by treatment with
11 ml of concentrated hydrochloric acid. Extraction of the crude D-(-)-
pantolactone yielded 15.29 grams. This material was then recrystallized from
7 ml of methyl isobutyl ketone and 7 ml hexane thereby yielding 9.77 grams
of D-(-)-pantolactone (37% of theory). The αD25 was -44.8°.
Into a vessel equipped with an agitator and reflux condenser are placed
approximately 52 parts by weight of α-hydroxy-β,β-di-methyl-γ-butyrolactone,
approximately 36 parts by weight of β-alanine, about 40 parts by weight of
diethylamine and about 100 parts by weight of anhydrous methanol. The
mixture is stirred and refluxed for about 12 hours until the reaction is
complete as evidenced by the dissolution of the β-alanine. To this resulting
mass is gradually added 8 parts by weight of calcium metal nodules or pellets
and refluxing continued until the metal is dissolved. The diethylamine and
alcohol are distilled off until the residue becomes viscous. The viscous residue
is dried under vacuum at 100°C. The solid residue recovered, as biologically
assayed, indicated a 91% yield of calcium pantothenate. | | Reactions | Pantothenic acid is a constituent of coenzyme A, which participates in numerous enzyme reactions. CoA was discovered as an essential cofactor for the acetylation of sulfanilamide in the liver and of choline in the brain. | | Brand name | Calpan (BASF); Pantholin
(Lilly). | | Therapeutic Function | Vitamin | | Biological Functions | Calcium D-Pantothenate accelerates the wound healing process by increasing the number of migrating cells, their distance and hence their speed. In addition, cell division is increased and the protein synthesis changed. Hence, higher quantities of pantothenate are locally required to enhance wound healing.
| | General Description | The first suggestion for the existance of vitamin B5 came from Carter et al. in 1930; although it was never characterized or isolated. This vitamin is synthesized by most green plants and microorganisms. Excellent sources of the vitamin are liver, egg yolk, whole grains, and fortified ready-to-eat cereals. However, as the original name implies, many foods contain sufficient pantothenic acid to supply dietary needs.
Chemically, pantothenic acid is considered to be aβ-alanine derivative of the asymmetric pantoic acid and thus shows asymmetry. Only the naturally occurring D(+)- stereoisomer (with R configuration) is biologically active and the L(-)-stereoisomer (with S configuration) is inactive. When its carboxylate functional group is attached through an amide linkage with β-mercaptoethylamine, it is known as pantetheine (also spelled pantotheine). The biologically active form of pantothenic acid, CoA, is formed when the terminal alcoholic function of pantetheine is attached to ADP 3'-phosphate. | | Biochem/physiol Actions | The calcium salts of panthenol are commonly used for pharmaceutical preparations. Pantothenate is a component of coenzyme A and is useful in its synthesis. Pantothenic acid is also involved in the synthesis of heme, cholesterol and fatty acids. Since vitamin B5 is found in all foods, its deficiency is not commonly observed. | | Clinical Use | The only therapeutic indication for pantothenic acid is intreatment of a known or suspected deficiency of this vitamin.Because of the ubiquitous nature of pantothenic acid, deficiencystates of this vitamin are only seen experimentally byuse of synthetic diets devoid of the vitamin, by use of thevitamin antagonist, ω-methylpantothenic, or both. In a 1991review, Tahiliani and Beinlich described that the mostcommon symptoms associated with pantothenic acid deficiencywere headache, fatigue, and a sensation of weakness.Sleep disturbances and gastrointestinal disturbances, amongothers, were also noted. The most likely setting for pantothenicacid deficiency is in the setting of alcoholism wherea multiple vitamin deficiency exists confounding the exactrole of the pantothenic acid deficiency as compared to theother vitamins. Because a deficiency of a single B vitamin israre, pantothenic acid is commonly formulated in multivitaminor B-complex preparations. | | Safety Profile | Moderately toxic by
intraperitoneal, subcutaneous, and
intravenous routes. Mildly toxic by
ingestion. A vitamin. See also CALCIUM
COMPOUNDS. When heated to
decomposition it emits toxic fumes of NOx. | | Purification Methods | The salt crystallises as needles from MeOH, EtOH or isoPrOH (with 0.5mol of isoPrOH). It is moderately hygroscopic. The S-benzylisothiuronium salt has m 151-152o (149o when crystallised from Me2CO). [Kagan et al. J Am Chem Soc 79 3545 1957, Wilson et al. J Am Chem Soc 76 5177 1954, Stiller & Wiley J Am Chem Soc 63 1239 1941, Beilstein 4 IV 2569.] |
| | Calcium D-Pantothenate Preparation Products And Raw materials |
|