|
|
| | Dexmedetomidine hydrochloride Basic information |
| Product Name: | Dexmedetomidine hydrochloride | | Synonyms: | 4-((S)-α,2,3-Trimethylbenzyl)imidazole monohydrochloride;DeMedetoMidine hydrochloride;(S)-4-(1-(2,3-diMethylphenyl)ethyl)-1H-iMidazole hydrochloride;1H-IMidazole,5-[(1S)-1-(2,3-diMethylphenyl)ethyl]-, hydrochloride (1:1);5-[(1S)-1-(2,3-dimethylphenyl)ethyl]-1H-imidazole,hydrochloride (1:1);Dexmedetomidine hydrochloride
4-((S)-alpha,2,3-Trimethylbenzyl)imidazole monohydrochloride;(S)-Medetomidine hydrochloride;Given dexmedetomidine hydrochloride | | CAS: | 145108-58-3 | | MF: | C13H17ClN2 | | MW: | 236.74 | | EINECS: | 682-047-2 | | Product Categories: | Inhibitors;API;145108-58-3 | | Mol File: | 145108-58-3.mol | 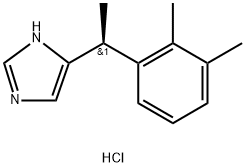 |
| | Dexmedetomidine hydrochloride Chemical Properties |
| Melting point | 156.5-157.5° | | alpha | +52.4° (c = 1 in water) | | density | 1.17 g/cm3 | | storage temp. | 2-8°C | | solubility | H2O: soluble20mg/mL, clear | | form | powder | | color | white to beige | | optical activity | [α]/D +48 to +58°, c = 1 in H2O | | Water Solubility | H2O: 20mg/mL, clear | | Merck | 14,2946 | | Stability: | Hygroscopic | | InChI | InChI=1/C13H16N2.ClH/c1-9-5-4-6-12(10(9)2)11(3)13-7-14-8-15-13;/h4-8,11H,1-3H3,(H,14,15);1H/t11-;/s3 | | InChIKey | VPNGEIHDPSLNMU-MERQFXBCSA-N | | SMILES | [C@@H](C1NC=NC=1)(C1C=CC=C(C)C=1C)C.Cl |&1:0,r| | | CAS DataBase Reference | 145108-58-3(CAS DataBase Reference) |
| | Dexmedetomidine hydrochloride Usage And Synthesis |
| Description | Dexmedetomidine was launched in the US as an i.v. infusion for sedation of initially
intubated and mechanically ventilated patients during treatment in an intensive care unit,
This imidazole derivative is the (S)-enantiomer of medetomidine that can be obtained via
fractional crystallization of the tartrate salt of the racemic mixture. Dexmedetomidine is a
full agonist of α2 adrenoceptors with 1300-fold selectivity versus α1 compared to the less
selective partial α2 agonist clonidine, a veterinary hypnotic. Dexmedetomidine is unique
compared with currently available sedatives in that it provides sedation, analgesia and
anxiolysis with the ability of patients to be easily awakened. Besides, it causes minimal
respiratory depression unlike other available drugs such as benzodiazepines or opioids.
Pharmacological studies in transgenic mice showed that the sedative, anesthetic and
analgesic effects of dexmedetomidine are specifically due to the stimulation of the a2A
subtype receptor. Like other α2 adrenoceptor agonists, dexmedetomidine can provoke
hypotension and bradycardia probably because of its non-selective action on peripheral
a2B subtype receptors in vascular smooth muscle. Dexmedetomidine is extensively
metabolized into methyl and glucuronide conjugates which are mainly eliminated by renal
excretion. It was found to be a CYP2D6 inhibitor but less potent than the clinically relevant
standard quinidine. | | Physical properties | Dexmedetomidine hydrochloride is a white or almost white powder that is freely soluble in water and has a pKa of 7.1. | | Originator | Orion (Finland) | | Uses | highly selective, potent α2-adrenoceptor agonist, analgesic, anxiolytic, bradycardic, hypotensive, sedative, hypothermic | | Uses | Dexmedetomidine hydrochloride has been used to study its effects on primary neonatal rat cardiomyocytes under hypoxic/reoxygenation conditions. It has also been used to study the effects of dexmedetomidine pretreatment on lipopolysaccharide-induced acute lung injury in mice. | | Definition | ChEBI: Dexmedetomidine hydrochloride is a medetomidine hydrochloride. It has a role as a sedative. It contains a dexmedetomidine. It is an enantiomer of a levomedetomidine hydrochloride. | | Brand name | Precedex | | benefits |
The chemical name of dexmedetomidine hydrochloride is (S) -4- [1- (2,3- 3,5-dimethylphenyl) ethyl] -1H- imidazole hydrochlorides. Dexmedetomidine hydrochloride has an anti-sympathetic town. The effect of quiet and analgesic, compared with U.S. support pyrimidine, with higher selectivity and a short half-life, can be clinically used for Intensive Care Therapy. Start intubation and calmness using a lung ventilator patient during treatment. Meanwhile, the medicine can also reduce narcotic consumption, improve hand incidence of hemodynamic stability, and reduce myocardial ischaemia in art.
| | Biological Activity | Active isomer of Medetomide (4-[1-(2,3-Dimethylphenyl)ethyl]-1H-imidazolehydrochloride ), a potent, highly selective α 2 -adrenoceptor agonist (K i values are 1.08 and 1750 nM for α 2 - and α 1 -adrenoceptors respectively). Displays greater selectivity over α 1 -adrenoceptors than clonidine and UK 14,304 (1620-, 220- and 300-fold respectively). Active in vivo ; displays hypotensive, bradycardic, sedative, anxiolytic, hypothermic and analgesic effects. | | Biochem/physiol Actions | Dexmedetomidine hydrochloride is a highly potent and selective α2-adrenergic agonist with analgesic and sedative properties, the (+)-isomer of medetomidine. Dexmedetomidine hydrochloride is used clinically as an anesthetic agent to reduce anxiety and tension and promote relaxation and sedation without causing respiratory depression. | | Side effects | Dexmedetomidine hydrochloride may cause serious side effects including:
low or high blood pressure,slow heart rate, and abnormal heart rate. | | Synthesis | In a 500mL round-bottomed flask, (S)-1 (18.6g, 93mmo1) is dissolved into 50mL dehydrated alcohol, and adding HC1/ ethanolic soln to adjust pH is 2~3, add THF solution 300mL, placement is spent the night, and now adularescent crystallization, filters, crude product is through ethyl acetate/ethyl alcohol recrystallization, filter, infrared drying oven is dried to obtain dexmedetomidine hydrochloride (20.1g, yield 91.5%).
| | storage | -20°C |
| | Dexmedetomidine hydrochloride Preparation Products And Raw materials |
|