|
|
| | bupivacaine hydrochloride Basic information |
| Product Name: | bupivacaine hydrochloride | | Synonyms: | Marcain;1-Butyl-N-(2,6-dimethylphenyl)-2-piperidinecarboxamide monohydrochloride;2',6'-Pipecoloxylidide, 1-butyl-, monohydrochloride;Einecs 241-917-8;Bupivacaine hydrochloride(Marcain);1-Butyl-N-(2,6-dimethylphenyl)piperidine-2-carboxamide Hydrochloride;2-Piperidinecarboxamide, 1-butyl-N-(2,6-dimethylphenyl)-, monohydrochloride;2',6'-Pipecoloxylidide, 1-butyl-, hydrochloride (6CI) | | CAS: | 18010-40-7 | | MF: | C18H28N2O.ClH | | MW: | 0 | | EINECS: | 241-917-8 | | Product Categories: | Marcain | | Mol File: | 18010-40-7.mol | 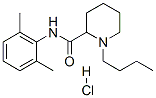 |
| | bupivacaine hydrochloride Chemical Properties |
| Melting point | 257°C(dec.)(lit.) | | storage temp. | 4°C, protect from light | | Water Solubility | Soluble in water | | solubility | ≥10.25 mg/mL in DMSO; ≥16.23 mg/mL in H2O with ultrasonic; ≥69.2 mg/mL in EtOH | | form | Solid | | color | White to Almost white | | Stability: | Hygroscopic |
| Hazard Codes | T+ | | Risk Statements | 26/27/28 | | Safety Statements | 22-36/37/39-45 | | RIDADR | UN 2811 6.1/PG 2 | | RTECS | TK6125000 | | HS Code | 2933.39.9200 | | HazardClass | 6.1 | | PackingGroup | II | | Toxicity | LD50 oral in rabbit: 18mg/kg |
| | bupivacaine hydrochloride Usage And Synthesis |
| Originator | Carbostesin,Astra,W. Germany,1967 | | Uses | Local anesthesic;Na+ channel blocker | | Definition | ChEBI: A racemate composed of equimolar amounts of dextrobupivacaine hydrochloride and levobupivacaine hydrochloride. The monohydrate form is commonly used as a local anaesthetic. | | Manufacturing Process | 121 parts by weight of 2.6-xylidine are heated with 400 parts of
diethylmalonate at 160°C for 1 hour, and the alcohol formed by the reaction is
allowed to distill off. Thereafter the reaction mass is cooled to 80°C, and 500
parts of alcohol are added. After cooling the dixylidide is sucked off, and the
alcohol solution with malonic ester monoxylidide is poured into 2,000 parts of
water. The monoxylidide precipitates, is filtered off and washed with water,
and recrystallized in diluted alcohol. Nitrosation thereafter takes place by
dissolving the dried monoxylidide in chloroform and by introducing nitrosyl
chloride at 0°C until the nitrosation is completed. The isonitrosomalonic ester
xylidide is filtered off and dried. Thereafter the reduction takes place with zinc
powder and formic acid at 90°-100°C.
The formic acid is distilled off, and the remainder dissolved in warm benzene
and washed with a bicarbonate solution to a neutral reaction. After the
benzene has been distilled off, the aminomalonic ester xylidide is obtained.
This is treated with an equal quantity of sodium ethylate and boiled with twice
the theoretical quantity of tetramethylene bromide in absolute alcohol.
After 6 hours of boiling, the sodium bromide formed is separated, and the
mixture is steamdistilled in order to remove the excess of tetramethylene
bromide. The remaining oil, which mainly consists of deltabromobutylaminomalonic
ester xylidide is separated from the water and boiled
with 3 parts of concentrated hydrochloric acid for 3 hours. Thereafter carbonfiltering
and evaporation to dryness under vacuum takes place. The residue is
dissolved in water, and the pH adjusted with sodium hydroxide to 5.5. The
solution is extracted twice with ether, and the water is made strongly alkaline
with sodium hydroxide.
The oil precipitates and is crystallized after a time. The crystals are separated
and dried under vacuum. The pipecolyl-2,6-xylidide produced is alkylated by
boiling for 10-20 hours with 0.6 part n-butylbromide in an n-butanol solution
in the presence of 0.5 part potassium carbonate. The potassium carbonate is
filtered off and the butanol is distilled off in vacuum. The residue is dissolved
in diluted hydrochloric acid and carbon treated, after which the base is
precipitated with sodium hydroxide in the form of white crystals, which are
filtered off and washed with water. The base obtained, which consists of N-n-butyl-pipecolyl-2,6-xylidide is sufficiently pure for the production of salts. | | Brand name | Marcaine (Hospira); Sensorcaine
(AstraZeneca). | | Therapeutic Function | Local anesthetic | | Biological Activity | bupivacaine hydrochloride is a local anaesthetic drug belonging to the amino amide group.bupivacaine hydrochloride is an effective local anesthetic agent. it has a rapid onset time, a high frequency of surgical anesthesia, a long duration, and a low incid |
| | bupivacaine hydrochloride Preparation Products And Raw materials |
|