- Tasimelteon
-

- $0.00 / 1KG
-
2025-03-25
- CAS:609799-22-6
- Min. Order: 1KG
- Purity: 99%min
- Supply Ability: 100kg
- Tasimelteon
-

- $0.00 / 1g
-
2025-01-13
- CAS:609799-22-6
- Min. Order: 1g
- Purity: More Than 99%
- Supply Ability: 100kg/Month
- Tasimelteon
-

- $38.00 / 10mg
-
2024-11-19
- CAS:609799-22-6
- Min. Order:
- Purity: 99.93%
- Supply Ability: 10g
|
| | Tasimelteon Basic information |
| Product Name: | Tasimelteon | | Synonyms: | Tasimelteon (1R-trans)-N-[[2-(2,3-Dihydro-4-benzofuranyl)cyclopropyl]methyl]propanamide;tasimelteon impurity A;tasimelteon;(1R-trans)-N-[[2-(2,3-Dihydro-4-benzofuranyl)cyclopropyl]methyl]propanamide;BMS 214778;MA 1;VEC 162;TasiMelteon/BMS214778 | | CAS: | 609799-22-6 | | MF: | C15H19NO2 | | MW: | 245.32 | | EINECS: | | | Product Categories: | Pharmaceuticals | | Mol File: | 609799-22-6.mol | 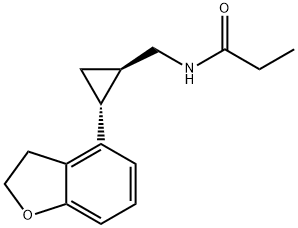 |
| | Tasimelteon Chemical Properties |
| Melting point | 78 °C | | Boiling point | 442.6±24.0 °C(Predicted) | | density | 1.145 | | storage temp. | 2-8°C | | solubility | Chloroform (Slightly), Methanol (Slightly) | | form | Solid | | pka | 16.43±0.46(Predicted) | | color | White to Off-White |
| | Tasimelteon Usage And Synthesis |
| Description | Tasimelteon, which is marketed by Vanda Pharmaceuticals as
Hetlioz� and developed in partnership with Bristol-Myers Squibb,
is a drug that was approved by the US FDA in January 2014 for
the treatment of non-24-hour sleep–wake disorder (also called
Non-24, N24 and N24HSWD). Tasimelteon is a melatonin MT1
and MT2 receptor agonist; because it exhibits a greater affinity to
the MT2 receptor than MT1, is also known as Dual Melatonin
Receptor Agonist.234 Two randomized controlled trials (phases II
and III) demonstrated that tasimelteon improved sleep latency
and maintenance of sleep with a shift in circadian rhythms, and
therefore has the potential to treat patients with transient insomnia
associated with circadian rhythm sleep disorders. Preclinical
studies showed that the drug has similar phase-shifting properties
to melatonin, but with less vasoconstrictive effects. | | Uses | Tasimelteon is a novel drug, used in the treatment of non-24 hour sleep-wake disorder. It helps to correct the circadian rhythm disorder often seen in patients who are visually impaired. | | Definition | ChEBI: A member of the class of 1-benzofurans that is propionamide in which one of the amide hydrogens is replaced by a [(1R,2R)-2-(2,3-dihydro-1-benzofuran-4-yl)cyclopropyl]methyl group. A melatonin receptor agonist used for the
treatment of non-24-hour sleep-wake disorder. | | Synthesis | Activation of commercial bis-ethanol 250 with 2.5 equivalents
of the Vilsmeier salt 251 followed by treatment with base resulted
an intramolecular cyclization reaction with the proximal phenol
and concomitant elimination of the remaining imidate to deliver
the vinylated dihydrobenzofuran 252 in 76% yield. Interestingly,
this reaction could be performed on multi-kilogram scale, required
no chromatographic purification, and generated environmentallyfriendly
DMF and HCl as byproducts. Sharpless asymmetric
dihydroxylation of olefin 252 delivered diol 253 in 86% yield and
impressive enantioselectivity (>99% ee). This diol was then activated
with trimethylsilyl chloride and then treated with base to generate epoxide 254. Next, a modified Horner¨CWadsworth¨C
Emmons reaction involving triethylphosphonoacetate (TEPA, 255)
was employed to convert epoxide 254 to cyclopropane 256.
The reaction presumably proceeds through removal of the acidic
TEPA proton followed by nucleophilic attack at the terminal epoxide
carbon. The resulting alkoxide undergoes an intramolecular
phosphoryl transfer reaction resulting in an enolate, which then
attacked the newly formed phosphonate ester in an SN2 fashion
resulting in the trans-cyclopropane ester, which was ultimately
saponified and re-acidified to furnish cyclopropane acid 256.
Conversion of this acid to the corresponding primary amide preceded
carbonyl reduction with sodium borohydride. The resulting
amine was acylated with propionyl chloride to furnish tasimelteon
(XXXI) as the final product in 86% yield across the four-step
sequence. 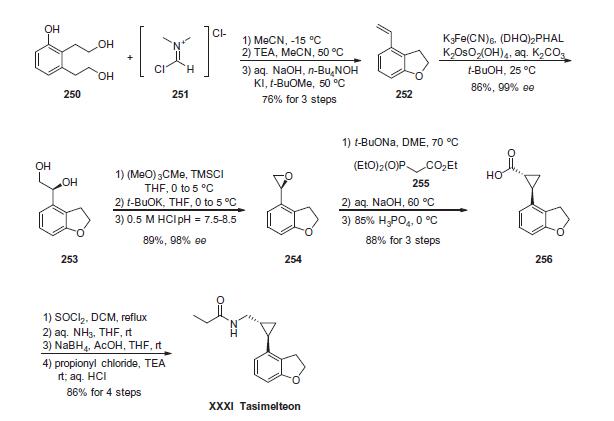
| | storage | Store at -20°C |
| | Tasimelteon Preparation Products And Raw materials |
|