| | Lead(II) nitrate Chemical Properties |
| Melting point | 470 °C (dec.)(lit.) | | Boiling point | 500℃[at 101 325 Pa] | | density | 1.00 g/mL at 20 °C | | bulk density | 1850kg/m3 | | storage temp. | Store below +30°C. | | solubility | H2O: soluble | | form | Solid | | Specific Gravity | 4.53 | | color | White | | PH | 3-4 (50g/l, H2O, 20℃) | | Odor | Odorless | | Water Solubility | 343 g/L | | Sensitive | Hygroscopic | | Hydrolytic Sensitivity | 0: forms stable aqueous solutions | | Merck | 14,5414 | | Dielectric constant | 37.7(0.0℃) | | Exposure limits | ACGIH: TWA 0.05 mg/m3
NIOSH: IDLH 100 mg/m3; TWA 0.050 mg/m3 | | Stability: | Stable. Strong oxidizer. Incompatible with combustible materials, organics, strong reducing agents. | | CAS DataBase Reference | 10099-74-8(CAS DataBase Reference) | | EPA Substance Registry System | Lead nitrate (Pb(NO3)2) (10099-74-8) |
| Hazard Codes | O,T,N,Xi,C | | Risk Statements | 61-8-20/22-33-50/53-62-52/53-36/38-51/53-35-41-34 | | Safety Statements | 53-45-60-61-17-26-36/37-36/37/39-39 | | RIDADR | UN 1469 5.1/PG 2 | | WGK Germany | 3 | | RTECS | OG2100000 | | TSCA | Yes | | HS Code | 2834 29 20 | | HazardClass | 5.1 | | PackingGroup | II | | Hazardous Substances Data | 10099-74-8(Hazardous Substances Data) | | Toxicity | guinea pig,LDLo,oral,500mg/kg (500mg/kg),BLOOD: PIGMENTED OR NUCLEATED RED BLLOD CELLSBEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD,Archiv fuer Hygiene und Bakteriologie. Vol. 125, Pg. 273, 1941. |
| | Lead(II) nitrate Usage And Synthesis |
| Overview | The lead nitrate appears as a white cubic crystal or monoclinic crystalline powder. It is soluble in water and liquid ammonia, being slightly soluble in the ethanol, being insoluble in concentrated nitric acid (produce protective film); it can react with concentrated hydrochloric acid or concentrated alkali chloride solution to form complex chlorinated lead acid or chlorinated lead acid salt; it has strong oxidizing property and may cause the risk of inducing combustion and explosion if mixed with organic matter, reducing agent and combustible substance such as sulfur and phosphorus or even subject to slightly friction. The dry lead nitrate can be subject to decomposition at around 205 ~223℃ while a temperature of 100℃ is enough to trigger the decomposition of wet lead nitrate. Its decomposition can emit toxic nitric oxide gas; This product is toxic with both swallowing and inhalation of the dust causing poisoning; It is better to be stored in a cool dry ventilated place; it should be subject to thoroughly cleaning before and after loading and unloading, isolated from heating, combustible substance, organic substance and other easily oxidized product. It should be kept away from food and metal powder; commonly used as mordant and oxidant, mainly used as the milk yellow pigment raw materials of glass and enamel; applied to industry such as paper, medicine, printing and dyeing, leather as well as for the manufacture of other lead salt. | | Preparation method | 1. Hydrometallurgy of galena
Weigh 0.4000 grams of galena in a dry 300ml beaker, add 15ml hydrochloric acid and steam at low temperature to evaporate 3~5 ml; add 10ml nitric acid-sulfate mixed acid, continue to heat and remove it until it is nearly dry. After slightly cooling, add 4 ml of sulfuric acid (1: 1) and wash the cup wall with distilled water to about 50ml. Boil for a few minutes, cool with water, add 10 mL of ethanol, let it stand for 1 hour and filter the precipitate using slow filter paper. Further use dilute sulfuric acid solution (2%) to wash for 3 to 4 times and finally wash with distilled water for additional 1 to 2 times. Discard the filtrate and put the precipitate and filter paper together back into the original beaker; add 30 mL acetic acid-sodium acetate buffer solution and boil until the lead sulfate is completely dissolved. After cooled, diluted with distilled water to 100ml; add about 0.1 gram of ascorbic acid, 2 to 3 drops of xylenol orange indicator and titrate with EDTA standard solution to until the solution color changes from pink to bright yellow which is the titration end.
2. take the metal lead as raw material, first make crude lead nitrate; after the hydrolysis of iron; apply lead replacement of copper, and then carry re-crystallization to obtain it.
(1) the production of crude lead nitrate
Lead ingot is first melted to make lead flower; at the case of slight excess amount of lead, it is subject to reaction with 20% to generate lead nitrate solution to until the reaction solution was light yellow. Filter while hot to remove impurities. The precipitation of the crystalline nitrate salt from the solution include precipitation and evaporation, two ways. 1. Salting-out method: Concentrated nitric acid is added under stirring, and lead nitrate is precipitated due to the common-ion effect. 2. Evaporation method: after applying acidification with nitric acid to the grass green color, apply heating and evaporation to the emergence of crystallization film; the lead nitrate crystals is precipitated out upon cooling; the crude lead nitrate is obtained after centrifugal separation.
(2) purification of crude lead nitrate
The main impurities in crude lead nitrate are iron and copper.
1. hydrolysis for iron removal
The impurity iron in the crude lead nitrate is present as an ionic Fe3 +. The pH value of aqueous solution of crude lead nitrate is 1~2; use ammonia to adjust to until the turbid phenomenon doesn’t disappear as more, the pH value at this time is 3~4. The Fe3 + should be completely hydrolyzed to form Fe (OH)3 precipitate to be removed.
2. replacement for copper removal
Carry out the replacement reaction for cooper removal at the condition of around 50℃.At this time, the solubility of the nitrate lead is 44% while the cooper content in the lead nitrate solution is about 0.025%. According to the Nernst equation, the replacement reaction can be carried out spontaneously. However, since the replacement reaction is carried out in nitric acid and lead nitrate solution (pH1) and the new replaced fine copper powder is more active. In order to prevent the re-dissolving of the nascent copper powder, the lead must be kept in excess. After the lead solution is added, slightly heat to maintain 10~15min, and then quickly filter, otherwise it is difficult to achieve the desired results.
(3). direct synthesis method using lead ore
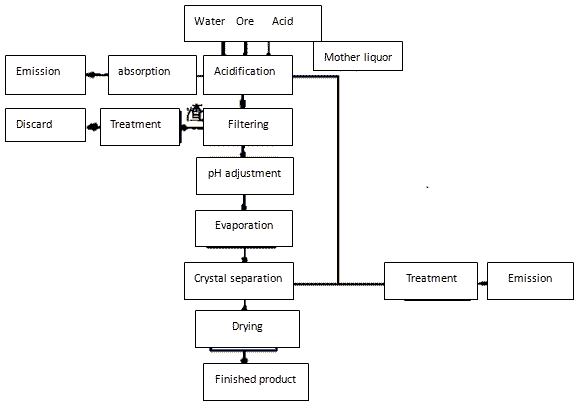
Lead sulfide ore is taken as raw material and subject to reaction with nitric acid under certain conditions to obtain the lead nitrate solution containing Zn2 +, Fe3 +, Cu2 + and other impurities. This solution, through impurity removal, concentration crystallization, can generate lead nitrate. The mother liquor, after adding potassium sulfate solution, can generate PbSO4 precipitation. After the separation of precipitate, the mother liquor is subject to treatment to remove Zn2 +, Fe3 +, Cu2 + and other impurities and can yield potassium nitrate after concentration. Lead sulfate, under the action of the catalyst, can yield metal lead after iron replacement. The lead then went back and dissolved for making lead nitrate. The reaction is as follows:
3PbS + 8HN03 = 3Pb (N03) 2 + 3S + 2N0 + 4H2O
2HNO3 + K2CO3 = 2KNO3 + CO2 + H2O
Fe3 + 30H = Fe (OH) 3 ↓
Pb (NO3) 2 + K2SO4 = PbSO4 ↓ + 2KN03
PbSO4 + Fe = FeSO4 + Pb
3Pb + 8HNO3 = 3Pb (NO3) 2 + 2NO + 4H2O
Cu2 + + 2OH = Cu (OH) 2 ?.
Zn2 + + 2OH-= Zn (OH) 2 ↓
4. Production of lead nitrate with fine ore powder as raw material
Mineral powder can have reaction after coming across acid, the main reaction occurs as follows:
3PbS + 8HN03 = 3Pb (N03) 2 + 3S ↓ + 2N0 ↑ + 4H2O
side reaction:
3ZnS + 8HNO3 = 3Zn (NO3) 2 + 2NO ↑ + 3S ↓ + 4H2O
3CuS + 8HNO3 = 3Cu (NO3) 2 + 2NO? + 3S? + 4H2O
FeS + 4HNO3 = Fe (NO3) 3 + NO + S + 2H2O
3Ag2S + 8HNO3 = 6AgNO3 + 2NO? + 3S? + 4H2O
As can be seen from the above reaction, with the leaching of lead in the ore, a large amount of impurities also enter the solution in the form of nitrate. Addition of certain amount of precipitant to the acid solution can enable the precipitation and further separation of ions such as Cu2 +, Fe3 + and Ag +.
Process flow chart is shown in Figure 1 below.
 | | Physical and chemical properties | It appears as colorless or white transparent cubic or monoclinic crystalline powder. The relative density is 4.53. It is soluble in water, liquid ammonia, slightly soluble in ethanol but insoluble in concentrated nitric acid (generate protective film). The aqueous solution was slightly acidic. At 470 °C, it is decomposed into lead oxide, nitrogen dioxide and oxygen. It can be mixed with flammable or fine powder to form an explosive mixture. It can be easily subject to hydrolysis in water to produce the white precipitate of the basic lead nitrate Pb (OH) NO3. This information is compiled by Ding Hong of Chemicalbook. | | Preparation and calibration | (1) Preparation:
Weigh 17g lead nitrate in 300ml beaker, add the appropriate amount of water, dissolved completely and transfer into a 1000ml volumetric flask, add 1ml nitric acid (1 +1) and dilute with water to the mark, mix uniformly.
(2) Calibration:
Take 20 mL 0.05mol/L EDTA solution and place in a 250mL Erlenmeyer flask. Add 15 mL 10% hexamethylenetetramine solution, 20mL of water and several drops of xylene. Use formulated lead nitrate solution for titration until the color changes from yellow to the red point. pipette Has been allocated to a good lead nitrate solution titration to yellow from the red which is the end point.

The standard concentration of lead nitrate solution (mL); C1-the standard concentration of EDTA solution; V-the volume of standard lead nitrate (mL); [Pb (NO3) (Mol/L): the concentration (mol/L) of the standard lead nitrate. | | Toxic effects | 1. Toxicity of lead nitrate on the mung bean seedlings
After cultivation of mung bean seedlings for five days, the half-inhibitory concentration (IC50) of leaf and root of the lead nitrate of mung bean seedlings were 276.33 and 6.47 mg/L, respectively. With the increasing of the concentration of the lead nitrate, the activity of the polyphenol oxidase (PPO) in the mung bean shoots exhibits inhibition-induction effect while the PPO activity in the root exhibits induction-inhibition effect. The activity of catalase (CAT) in leaf and root of mung bean showed induction-inhibition effect; the chlorophyll content in mung bean shoots exhibits inhibitory effect.
2. Toxicity of the lead nitrate on the chromosome of onion root tip
In the dose range, with the increased concentration of the aqueous solution of the lead nitrate and the prolonged treatment time of the toxic staining, the mitotic index of the onion root tip cells decrease gradually, the chromosome aberration rate increases and the micronucleus rate also increases gradually. The mitotic index of onion root tip cells decreased gradually, which may be due to the inhibition of the synthesis of triggering protein required for entry into S phase, leading to delayed cell cycle and further decrease of cell division index. The nuclear rupture may be due to that the lead nitrate destroy the nuclear pore complex protein. Since the chromosome get re-connected after breakage, it undergoes aberrations, duplications, inversions, and translocations, resulting in the chromosomal structural variation and further emergence of the chromosome bridge. The chromosomal adhesion, chromosomal lag and chromosomal asymmetrical segregation may be due to disruption of microtubule assembly by lead nitrate, thus the spindle was interrupted so it can not guide chromosomes. | | Precautions | This product is toxic with both swallowing and inhalation of its dust being able to causing poisoning. It can be dissolved in water. It should be stored in a cool dry and ventilated place. It should be thoroughly cleaned before and after the loading and unloading, isolated from heating, combustible product, organic and other easily oxidized products. It should be kept away from food and metal powder. Any spilled lead nitrate should be immediately removed and disposed. Upon fire, we can use mist water and sand. A small amount fire of lead nitrate can be washed with plenty of water. For example, when a large number of lead nitrate get fire, nitrate may melt and melt, avoid using water for fire extinguishing to prevent the spray of a large amount of molten lead nitrate. After subjecting to lead nitrate poisoning, we can drink milk upon, protein and trace amount of magnesium sulfate as well as plenty of water upon mild poisoning. | | Solubility in water (g / 100ml) | Solubility in water (g/100ml) at different temperatures (° C):
37.5 g/0 ° C; 46.2 g/10 ° C; 54.3 g/20 ° C; 63.4 g/30 ° C; 72.1 g /
91.6 g/60 ° C; 111 g/80 ° C; 133 g/100 ° C | | Toxicity | Lead nitrate is toxic to human being, in particular, can cause change on the nervous system, blood and blood vessels. It has large impact on protein metabolism, cell energy balance and the genetic system. It can appear of lead line in the gingival margin (mainly on the anterior teeth), the so-called "lead color"-the skin exhibits soil gray. The lead in the urine is considered to be the symptoms of carrying lead.
Upon mistakenly eating lead nitrate lead that causes acute poisoning, we can first apply 1% to 2% magnesium sulfate solution for gastric lavage for intravenous injection of 10 mL 10% sodium thiosulfate; upon chronic poisoning, we can administrate sodium calcium.
The maximal allowable concentration of lead and inorganic lead compounds is 0.01 mg/m3. The average concentration in working day is 0.007 mg/m3.
There are lead vapor, nitrogen oxide gas and lead nitrate existing during the production process, these substances are toxic so the equipment should be closed and the production environment should be well ventilated. During the operation, the worker should wear masks. If the lead vapor concentration is high, the worker should use the filter type gas masks. During the working hours, it is prohibited to eat or smoke in the workplace and the workers should wear protective overalls and other labor insurance products. After the work, the worker should take hot water shower, gargle and brush teeth before meal and after work. Have regular physical examination and should give health food. | | Uses | 1. It can be used as analysis reagents, oxidizers and photo sensitizer, also used for plating and so on
2. It can be used for making lead salt as well as applied to medicine, printing and dyeing, matches, pigments, dyes, explosives, tanning, plate making, pyrotechnics and other industries.
3. Glass lined industry applies it for the manufacture of milk yellow pigment. Paper industry use it as the yellow pigment of paper. Dyeing industry use it as mordant. Inorganic industry applies it for the manufacture of other lead salts and lead dioxide. The pharmaceutical industry uses it for the manufacture of astringents. Benzene industry use it for tanning agents. Photographic industry use it as a photo sensitizer. Mining industry applies it as ore flotation agent. In addition, it is also used for the production of matches, pyrotechnics, explosives, oxidants, and analytical chemical reagents. | | Hazards & Safety Information | Category Oxidant
Toxicity classification highly toxic
Acute toxicity Intraperitoneal-rat LDL0: 270 mg/kg; Abdominal-mouse LD50: 74 mg/kg
Explosive hazardous properties it is explosive when being mixed, heated, impacted and rubbed by reducing agent, sulfur and phosphorus.
Combustible Hazardous properties it is combustible when mixed with organic product, reducing agent, combustible sulfur and phosphorous; Combustion produces toxic nitrogen oxides and lead-containing fumes
Storage and transportation characteristics Treasury: ventilated, low-temperature and dry; light loading and unloading; store separately from organic matter, reducing agent, sulfur, phosphorus and other combustible materials.
Fire extinguishing agent mist water, sand
Occupational Standard TWA 0.15 mg (lead)/m³ | | Chemical Properties | Lead nitrate, Pb(NO3)2, sp gr 4.53, forms cubic or monoclinic colorless crystals. Above 205 °C, oxygen and nitrogen dioxide are driven off, and basic lead nitrates are formed. Above 470 °C, lead nitrate is decomposed to lead monoxide and Pb3O4. Lead nitrate is highly soluble in water (56.5 g/100 mL at 20 °C; 127 g/100 mL at 100 °C), soluble in alkalies and ammonia, and fairly soluble in alcohol (8.77 g/ 100 mL of 43% aqueous ethanol at 22 °C). Lead nitrate is readily obtained by dissolving metallic lead, lead monoxide, or lead carbonate in dilute nitric acid. Excess acid prevents the formation of basic nitrates, and the desired lead nitrate can be crystallized by evaporation. | | Chemical Properties | The lead nitrate appears as a white cubic crystal or monoclinic crystalline powder. It is soluble in water and liquid ammonia, being slightly soluble in the ethanol, being insoluble in concentrated nitric acid (produce protective film); it can react with concentrated hydrochloric acid or concentrated alkali chloride solution to form complex chlorinated lead acid or chlorinated lead acid salt; it has strong oxidizing property and may cause the risk of inducing combustion and explosion if mixed with organic matter, reducing agent and combustible substance such as sulfur and phosphorus or even subject to slightly friction. The dry lead nitrate can be subject to decomposition at around 205 ~223 while a temperature of 100 is enough to trigger the decomposition of wet lead nitrate. Its decomposition can emit toxic nitric oxide gas. | | Physical properties | Colorless cubic or monoclinic crystals; refractive index 1.782; density 4.53 g/cm3 at 20°C; decomposes at 470°C; soluble in cold water; very soluble in boiling water 127 g/100 mL at 100°C; also soluble in caustic soda, caustic potash and ammonia solution, and moderately soluble in alcohol. | | Uses | Lead nitrate is used in many industrial processes, ranging from ore processing to pyrotechnics to photothermography. Thus lead nitrate is used as a flotation agent in titanium removal from clays; in electrolytic refining of lead; in rayon delustering; in red lead manufacture; in matches, pyrotechnics, and explosives; as a heat stabilizer in nylon; as a coating on paper for photothermography; as an esterification catalyst for polyesters; as a rodenticide; as an electroluminescent mixture with zinc sulfide; as a means of electrodepositing lead dioxide coatings on nickel anodes; and as a means of recovering precious metals from cyanide solutions. | | Uses | manufacture of matches and special explosives; as mordant in dyeing and printing on textiles; mordant for staining horn, mother-of-pearl; oxidizer in dye industry; sensitizer in photography; process engraving. | | Uses | Lead(II) nitrate is employed in industrial applications such as heat stabilization in nylon and polyesters and coatings of photothermographic paper. It is utilized to improve the leaching process in the gold cyanidation. It finds applications as a lead paint as well as a source of dinitrogen tetroxide. As an oxidant, it is used in the oxidation of benzylic halides to aldehydes in organic synthesis. It is also used in the preparation of dithiocarbamates from isothiocyanates. It acts as a scavenger of bromide in nucleophilic substitution reactions. | | Preparation | Lead nitrate is prepared by dissolving lead metal, lead monoxide or lead carbonate in excess dilute nitric acid followed by evaporation of and/or cooling the solution for crystallization. | | General Description | Lead dinitrate is a white crystalline solid. The material is soluble in water. Lead dinitrate is noncombustible but Lead dinitrate will accelerate the burning of combustible materials. If large quantities of the material are involved in the fire an explosion may result. Prolonged exposure of the material to fire or heat may result in an explosion. Toxic oxides of nitrogen are produced in fires involving Lead dinitrate. | | Air & Water Reactions | Water soluble. | | Reactivity Profile | Mixtures of metal/nonmetal nitrates with alkyl esters may explode because of the formation of alkyl nitrates; mixtures of nitrate with phosphorus, tin (II) chloride or other reducing agents may react explosively [Bretherick 1979. p. 108-109]. An explosion of guanidine nitrate demolished an autoclave built to withstand 50 atmospheres, in which Lead dinitrate was being made from ammonium thiocyanate and Lead dinitrate [C. Angew. Chem. 49:23. 1936]. | | Hazard | The toxic effects are greater than other lead salts because lead nitrate is more soluble. Moderately toxic by ingestion and other routes of exposure. The compound also is an irritant to eye, skin, and mucous membranes. | | Health Hazard | Early symptoms of lead intoxicatin via inhalation or ingestion are most commonly gastrointestinal disorders, colic, constipation, etc.; weakness, which may go on to paralysis, chiefly of the extensor muscles of the wrists and less often the ankles, is noticeable in the most serious cases. Ingestion of a large amount causes local irritation of the alimentary tract; pain, leg cramps, muscle weakness, paresthesias, depression, coma, and death may follow in 1 or 2 days. Contact with eyes causes irritation. | | Flammability and Explosibility | Non flammable | | reaction suitability | reagent type: catalyst
core: lead | | Industrial uses | This is a white to colorless fine crystalline compound, extremely soluble in water (34%
at 20 °C). Commercial production is based on dissolution of lead metal or lead compounds
in nitric acid (36–40% solution). Lead nitrate is considered to be an activator in
mineral processing. Although lead may activate sphalerite, similar to CuSO4, the use of
Pb(NO3)2 is limited to the activation of stibnite during beneficiation of antimony ores.
Lead nitrate is the most widely used chemical in cyanidation of precious metals as an
accelerator. | | Purification Methods | Precipitate it twice from a hot (60o) concentrated aqueous solution by adding HNO3. The precipitate is sucked dry on a sintered-glass funnel, then transferred to a crystallising dish which is covered by a clock glass and left in an electric oven at 110o for several hours [Beck et al. Trans Faraday Soc 55 331 1959]. After two recrystallisations of ACS grade, no metals above 0.001ppm were detected. |
| | Lead(II) nitrate Preparation Products And Raw materials |
|