- Doxepin
-

- $1980.00 / 50mg
-
2024-10-28
- CAS:1668-19-5
- Min. Order:
- Purity:
- Supply Ability: 10g
- DOXEPIN USP/EP/BP
-
- $1.10 / 1g
-
2021-07-27
- CAS:1668-19-5
- Min. Order: 1g
- Purity: 99.9%
- Supply Ability: 100 Tons min
- DOXEPIN
-

- $7.00 / 1KG
-
2020-01-10
- CAS:1668-19-5
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 100KG
|
| | DOXEPIN Basic information |
| Product Name: | DOXEPIN | | Synonyms: | (3Z)-3-Dibenzo[b,E]oxepin-11(6H)-ylidene-N,N-dimethyl-1-propanamine;11-(3-(dimethylamino)propylidene)-6h-dibenz(b,e)0xepine;11-(3-dimethylamino-propyliden)-6,11-dihydro-dibenz(b,e)oxipin;11-(3-Dimethylaminopropylidene)-6,11-dihydrodibenz(b,e)oxipin;3-dibenz(b,e)oxepin-11(6h)-ylidene-n,n-dimethyl-1-propanamin;Quitaxon;1-Propanamine, 3-dibenzb,eoxepin-11(6H)-ylidene-N,N-dimethyl-;11-(3-Dimethylaminopropylidene)-6,11-dihydrodibenz[b,e]oxepin | | CAS: | 1668-19-5 | | MF: | C19H21NO | | MW: | 279.38 | | EINECS: | 214-966-8 | | Product Categories: | Heterocyclic Compounds;Neurochemicals | | Mol File: | 1668-19-5.mol | 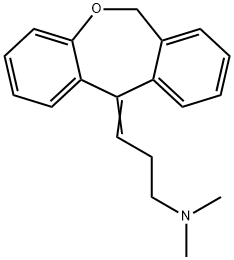 |
| | DOXEPIN Chemical Properties |
| Melting point | 187-189°C | | Boiling point | bp0.03 154-157°; bp0.2 260-270° | | density | 1.0594 (rough estimate) | | refractive index | 1.5000 (estimate) | | storage temp. | Sealed in dry,2-8°C | | pka | 9.40±0.28(Predicted) | | Water Solubility | 31.57mg/L(25 ºC) | | EPA Substance Registry System | Doxepin (1668-19-5) |
| | DOXEPIN Usage And Synthesis |
| Originator | Sinequan,Pfizer,US,1969 | | Uses | Used clinically to treat anxiety and depression. Antidepressant | | Definition | ChEBI: A dibenzooxepine that is 6,11-dihydrodibenzo[b,e]oxepine substituted by a 3-(dimethylamino)propylidene group at position 11. It is used as an antidepressant drug. | | Manufacturing Process | (A) Preparation of 3-bromopropyltriphenylphosphonium bromide:
Triphenylphosphine, 1.0 kg, and 770 grams of 1,3-dibromopropane are
dissolved in 2.0 liters of xylene and the solution is stirred under a nitrogen
atmosphere at 130°C. After 20 hours the mixture is cooled, and the crystalline
product, which precipitates, is collected and washed with 20 liters of benzene.
After drying in vacuo the product weighs 1,578 grams, MP 229°-230°C;titration for bromide ion: Found, 17.1%; calculated, 17.2%.
(B) Preparation of 3-dimethylaminopropyltriphenylphosphonium bromide
hydrobromide: A solution of 595 grams of anhydrous dimethylamine and
1,358 grams of 3-bromopropyl-triphenylphosphonium bromide in 4 liters of
ethanol is warmed to 70°C until solution is complete and the solution then is
allowed to stand at room temperature for 20 hours. Volatile components are
removed by distillation in a vacuum and the residue is suspended in 2.0 liters
of ethanol and is redistilled to remove excess amine. The residue is dissolved
in 3.0 liters of warm ethanol and gaseous hydrogen bromide is passed into
the solution until the mixture is acidic. After filtration the solution is
concentrated to a volume of 3.0 liters, is cooled, whereupon the product
precipitates, and the precipitate is collected; it weighs 1,265 grams, MP 274-
281°C. Recrystallization from ethanol raises the MP to 280.5°-282.5°C.
Bromide ion titration: Found, 31.2%; calculated 31.3%.
(C) Preparation of doxepin: 1,530 grams of the product from step (B) is
suspended in 4.5 liters dry tetrahydrofuran and 6.0 mols of butyl lithium in
heptane is added during 1 hour. After an additional 30 minutes, 483 grams of
6,11-dihydrodibenz-(b,e)oxepin-11-one, prepared as described in Belgian
Patent 641,498, is added to the deep red solution and the reaction was
maintained at reflux for 10 hours. Water, 500 ml, is added at room
temperature and the solvent is removed in vacuo. The crude residue is treated
with 10% hydrochloric acid until acidic (pH 2) and then 1.5 liters benzene is
added. After stirring, the mixture separates into 3 phases (an insoluble
hydrochloride salt product phase, an aqueous phase and an organic phase).
The benzene layer is removed by decantation and the remaining mixture is
rendered basic with 10% sodium hydroxide solution and is extracted with
three 1,500 ml portions of benzene. The benzene extracts are washed, then
dried with anhydrous sodium sulfate and concentrated in a vacuum leaving a
residue of 1,530 grams, gas and thin layer chromatography analysis show this
to be a cis/trans mixture (approx. 4:l) of 11-dimethylaminopropylidene-6,11-
dihydrodibenz-(b,e)oxepin (90% yield). This mixture has substantially more
activity pharmacologically than the cis/trans mixture obtained by the Grignard
route disclosed in the Belgian Patent 641,498. This base is then converted to
the hydrochloride with HCl. | | Brand name | Apo-doxepin;Co dox;Deptran;Doksapan;Dolat;Doxal;Doxedyn;Doxepin hcl;Gilex;Novo-doxepin;Novoxapin;Sinequan;Sinquan concentrate;Sinquane;Tolllluan;Triadapin;Zonalon. | | Therapeutic Function | Tranquilizer | | World Health Organization (WHO) | Doxepin, a tricyclic antidepressant was introduced in 1964 for the
management of endogenous depression. Much of the adverse effects are caused
by its antimuscarinic actions. These include dry mouth, cardiac arrhythmias,
central nervous system disturbances, blood disorders and risk of suicide. The risk
of suicide and dangers related to overdosage led the Norwegian Medicines Control
Authority to put the higher strength formulation under prescribing restriction in
1992. The risk of death following overdosage is apparently higher for products
containing tricyclic compounds as compared with nontricyclic products. | | Biological Activity | Highly potent H 1 histamine receptor antagonist (K d = 310 pM) and tricyclic antidepressant. Also binds to the H 4 histamine receptor (pK i = 6.79). | | Contact allergens | This benzoxepin tricylcic drug has antidepressant, anticholinergic,
antiitching, and antihistamine properties.
After oral use, it has been developed as a topical antiitching
agent. Allergic contact dermatitis is not infrequent. |
| | DOXEPIN Preparation Products And Raw materials |
|