|
|
| | 3,4-DIHYDROQUINOLIN-2(1H)-ONE Basic information |
| Product Name: | 3,4-DIHYDROQUINOLIN-2(1H)-ONE | | Synonyms: | DIHYDROCARBOSTYRIL;3,4-DIHYDRO-2(H)-QUINOLINE;3,4-DIHYDRO-2(1H)-QUINOLONE;1,2,3,4-Tetrahydroquinolin-2-one;2-OXO-1,2,3,4-TETRAHYDROQUINOLINE;2-OXO-1,3,4-TRIHYDROQUINOLINE;dihydro-α-quinolone;o-aminohydrocinnamic acid lactam | | CAS: | 553-03-7 | | MF: | C9H9NO | | MW: | 147.17 | | EINECS: | 621-863-5 | | Product Categories: | Quinolines, Isoquinolines & Quinoxalines;Heterocycles;pharmacetical;API intermediates | | Mol File: | 553-03-7.mol | 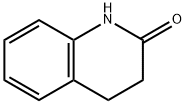 |
| | 3,4-DIHYDROQUINOLIN-2(1H)-ONE Chemical Properties |
| Melting point | 165-167 °C (lit.) | | Boiling point | 267.28°C (rough estimate) | | density | 1.1135 (rough estimate) | | refractive index | 1.5200 (estimate) | | storage temp. | Keep in dark place,Sealed in dry,Room Temperature | | solubility | DMF: 30 mg/ml,DMSO: 30 mg/ml,DMSO:PBS (pH 7.2) (1:6): 0.14 mg/ml | | form | Crystalline Powder | | pKa | 14.76±0.20(Predicted) | | color | Off-white | | Water Solubility | Slightly soluble in water. | | Sensitive | Light Sensitive | | Merck | 13,4800 | | CAS DataBase Reference | 553-03-7(CAS DataBase Reference) |
| Hazard Codes | Xn,Xi | | Risk Statements | 22-36/37/38-43 | | Safety Statements | 26-36/37 | | RIDADR | 2811 | | WGK Germany | 2 | | Hazard Note | Irritant | | HazardClass | 6.1(b) | | PackingGroup | III | | HS Code | 29337900 |
| | 3,4-DIHYDROQUINOLIN-2(1H)-ONE Usage And Synthesis |
| Chemical Properties | Off-white crystalline powder | | Uses | 3,4-Dihydro-2(1H)-quinolinone may be employed as medium supplement in the culture medium of Pseudomonas ayucida during enrichment culture experiments. | | Uses | 3,4-Dihydro-2-(1H)-quinolinone may be employed as medium supplement in the culture medium of Pseudomonas ayucida during enrichment culture experiments. It is used to prepare potent bicyclic peptide deformylase inhibitors with antibacterial effects. It is also used to synthesize substituted iminopiperidines as inhibitors of human nitric oxide synthase isoforms. | | Definition | ChEBI: 3,4-Dihydro-2(1H)-quinolinone is a member of quinolines. | | Synthesis Reference(s) | Journal of the American Chemical Society, 89, p. 7131, 1967 DOI: 10.1021/ja01002a061
Tetrahedron Letters, 36, p. 125, 1995 DOI: 10.1016/0040-4039(94)02191-D | | General Description | Series of 3,4-dihydro-2(1H)-quinolinone derivatives having sigma-1 receptor (σ1R) antagonist activity have been synthesized. 3,4-dihydro-2((1)H)-quinolinones have been synthesized using N-(1′-alkoxy)cyclopropyl-2-haloanilines as starting reagent. Cyclopropane ring expansion in the presence of palladium catalyst is the major step involved in the synthesis. Library of 3,4-dihydro-2(1H)-quinolinones have been synthesized through the rearrangement of β-lactam intermediates on the solid-phase |
| | 3,4-DIHYDROQUINOLIN-2(1H)-ONE Preparation Products And Raw materials |
|