- (-)-P-MENTHA-1,5-DIENE
-
- $20.00 / 1KG
-
2024-08-12
- CAS:4221-98-1
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 52000KG
|
| | (-)-P-MENTHA-1,5-DIENE Basic information |
| Product Name: | (-)-P-MENTHA-1,5-DIENE | | Synonyms: | (R)-5-isopropyl-2-methylcyclohexa-1,3-diene;1,3-Cyclohexadiene, 2-methyl-5-(1-methylethyl)-, (5R)-;R-(-)-A-PHELLANDRENE WITH GC;(-)-α-phellandrene;(R)-2-methyl-5-(1-methylethyl)-1,3-cyclohexadiene;(-)-p-Mentha-1,5-diene, (R)-5-Isopropyl-2-methyl-1,3-cyclohexadiene;PETUNIDIN CHLORIDE(SH);(R)-()-α-Phellandrene,()-p-Mentha-1,5-diene, (R)-5-Isopropyl-2-methyl-1,3-cyclohexadiene | | CAS: | 4221-98-1 | | MF: | C10H16 | | MW: | 136.23 | | EINECS: | 224-167-6 | | Product Categories: | Biochemistry;Monocyclic Monoterpenes;Terpenes | | Mol File: | 4221-98-1.mol | 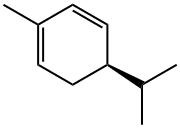 |
| | (-)-P-MENTHA-1,5-DIENE Chemical Properties |
| alpha | D20 -217° | | Boiling point | 171-174 °C(lit.) | | density | 0.844 g/mL at 20 °C(lit.) | | vapor pressure | 2.35hPa at 20℃ | | refractive index | n20/D 1.475 | | Fp | 49 °C | | storage temp. | 2-8°C | | solubility | Benzene (Sparingly), Ethyl Acetate (Slightly) | | form | clear liquid | | color | Colorless to Light yellow to Light orange | | Odor | at 100.00 %. terpene spicy medicinal | | Odor Type | terpenic | | optical activity | [α]20/D 225±5°, c = 10% in diethyl ether | | Water Solubility | 4.37mg/L at 20℃ | | Merck | 14,7200 | | BRN | 2497824 | | LogP | 5.74 at 21.6℃ | | CAS DataBase Reference | 4221-98-1(CAS DataBase Reference) | | EPA Substance Registry System | 1,3-Cyclohexadiene, 2-methyl-5-(1-methylethyl)-, (5R)- (4221-98-1) |
| Risk Statements | 10 | | RIDADR | UN 2319 3/PG 3 | | WGK Germany | 3 | | F | 23 | | HS Code | 2902.19.0050 | | HazardClass | 3 | | PackingGroup | III |
| | (-)-P-MENTHA-1,5-DIENE Usage And Synthesis |
| Chemical Properties | (?)-α-Phellandrene is a colorless
liquid with a citrus odor and a slight peppery note. It is isolated, for example, from
Eucalyptus dives oil. Pinenes are widespread, naturally occurring terpene hydrocarbons. The α- and
β-forms occur in varying ratios in essential oils. | | Uses | (R)-(-)-α-Phellandrene undergoes [4+2] cycloaddition with 2-naphthyl acetylenecarboxylate to form a chiral diene, which can act as a ligand for the rhodium-catalyzed asymmetric addition reactions. It may be used to synthesize (1S,5R)-5-(1-methylethyl)-2-methylidene-3-cyclohexen-1-yl hydroperoxide, which on reduction forms trans-yabunikkeol. | | Definition | ChEBI: (-)-alpha-phellandrene is the (R)-(-)-stereoisomer of alpha-phellandrene. It is an enantiomer of a (+)-alpha-phellandrene. | | General Description | (R)-(-)-α-Phellandrene is a monoterpene derivative. | | Flammability and Explosibility | Flammable | | Purification Methods | Purify it by gas chromatography on an Apiezon column. Also purify it by steam distillation (with 0.5% hydroquinone), then re-distil it through a 50 plate bubble cap column b 72-72.5o/22mm [Pines & Eschinazi J Am Chem Soc 77 6318 1955]. UV: max 263nm ( 3,345) in octane. [Read & Storey J Chem Soc 2770 1930, Beilstein 5 III 341, 5 IV 436.] | | Toxics Screening Level | The screening level is being set at the default of 0.1 μg/m3 with annual averaging. |
| | (-)-P-MENTHA-1,5-DIENE Preparation Products And Raw materials |
|