- Sodium trifluoroacetate
-

- $0.00 / 25kg
-
2025-03-21
- CAS:2923-18-4
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 50000KG/month
|
| | Sodium trifluoroacetate Basic information |
| Product Name: | Sodium trifluoroacetate | | Synonyms: | SodiuM trifluoroacetate, 98%, 98%;Sodium 2,2,2-trifluoroethanoate, Trifluoroacetic acid, sodium salt;Trifluoroacetic acid, sodiuM salt, 97% 100GR;Trifluoroacetic acid, sodiuM salt, 97% 25GR;sodiumperfluoroacetate;trifluoro-aceticacisodiumsalt;TRIFLUOROACETIC ACID SODIUM SALT;SODIUM TRIFLUOROACETATE | | CAS: | 2923-18-4 | | MF: | C2F3NaO2 | | MW: | 136.01 | | EINECS: | 220-879-6 | | Product Categories: | metal acetate salt;top | | Mol File: | 2923-18-4.mol | 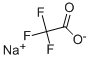 |
| | Sodium trifluoroacetate Chemical Properties |
| Melting point | 205-207 °C (dec.) (lit.) | | density | 1.49 g/mL (lit.) | | storage temp. | Keep in dark place,Inert atmosphere,Room temperature | | solubility | H2O: 1 M at 20 °C, clear, colorless | | form | Crystalline Powder | | Specific Gravity | 1.49 | | color | White | | PH | 7 (H2O, 20℃) | | Water Solubility | 625 g/L (25 ºC) | | Sensitive | Hygroscopic | | Hydrolytic Sensitivity | 6: forms irreversible hydrate | | BRN | 3597949 | | Stability: | Hygroscopic | | InChIKey | UYCAUPASBSROMS-UHFFFAOYSA-M | | LogP | 1.238 (est) | | CAS DataBase Reference | 2923-18-4(CAS DataBase Reference) | | EPA Substance Registry System | Acetic acid, trifluoro-, sodium salt (2923-18-4) |
| Hazard Codes | Xi | | Risk Statements | 36/37/38 | | Safety Statements | 26-36-37/39 | | RIDADR | 2811 | | WGK Germany | 1 | | RTECS | AK0250000 | | F | 3-10 | | Hazard Note | Irritant/Hygroscopic | | TSCA | T | | HazardClass | 6.1 | | PackingGroup | II | | HS Code | 29159080 |
| | Sodium trifluoroacetate Usage And Synthesis |
| Chemical Properties | Sodium trifluoroacetate is an organic metal compound with the chemical formula CF3COONa. It is a white crystalline powderr that easily absorbs moisture. This compound has the ability to add a trifluoromethyl group to halogenated aromatic hydrocarbons. | | Uses | Sodium trifluoroacetate is used in acid catalyzed reactions. It is also used as an intermediate for active pharmaceutical ingredients (API). Further, it acts as a precursor for the trifluoromethylation of aldehydes using copper(I) halide as a catalyst. | | Preparation | It can be obtained by reacting trifluoroacetic acid with sodium carbonate or sodium hydroxide. | | Purification Methods | A possible contaminant is NaCl. The solid is treated with CF3CO2H and evaporated twice. Its solubility in CF3CO2H is 13.1% at 29.8o. The residue is crystallised from dilute EtOH, and the solid is dried in vacuum at 100o. [Hara & Cady J Am Chem Soc 76 4285 1954.] It can be precipitated from EtOH by adding dioxane, then recrystallising several times from hot absolute EtOH. Dry it at 120-130o/1mm. [Beilstein 2 IV 461.] |
| | Sodium trifluoroacetate Preparation Products And Raw materials |
|