- Fludioxonil
-

- $100.00 / 25Kg/Bag
-
2025-03-07
- CAS:131341-86-1
- Min. Order: 25Kg/Bag
- Purity: 99.99%
- Supply Ability: 200ton
- FLUDIOXONIL
-

- $0.00 / 25Kg/Bag
-
2025-03-07
- CAS:131341-86-1
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 20ton
- Fludioxonil
-

- $0.00 / 25kg
-
2025-02-21
- CAS:131341-86-1
- Min. Order: 10kg
- Purity: 98
- Supply Ability: 20000
Related articles - Safety of Fludioxonil
- Fludioxonil is a new type of pyrrole non-systemic, contact-killing broad-spectrum fungicide. As a seed treatment fungicide, th....
- Oct 29,2021
|
| | Fludioxonil Chemical Properties |
| Melting point | 199.4° | | Boiling point | 420.4±45.0 °C(Predicted) | | density | 1.55±0.1 g/cm3(Predicted) | | vapor pressure | 3.9 x 10-7 Pa (25 °C) | | storage temp. | Inert atmosphere,Room Temperature | | solubility | DMSO: Slightly Soluble,Methanol: Slightly Soluble | | Water Solubility | 1.8 mg l-1 in water at 25 °C | | pka | 14.10±0.50(Predicted) | | form | Solid | | color | White to off-white | | BRN | 8393936 | | InChIKey | MUJOIMFVNIBMKC-UHFFFAOYSA-N | | EPA Substance Registry System | Fludioxonil (131341-86-1) |
| Hazard Codes | N | | Risk Statements | 50/53 | | Safety Statements | 60-61 | | RIDADR | 3077 | | WGK Germany | 3 | | RTECS | UX9347525 | | HazardClass | 9 | | PackingGroup | III | | HS Code | 29349990 | | Hazardous Substances Data | 131341-86-1(Hazardous Substances Data) | | Toxicity | LD50 in rats (mg/kg): >2000 orally; >2000 dermally; LC50 (4 hr) in rats: >2600 mg/m3 (Gehmann) |
| | Fludioxonil Usage And Synthesis |
| Description | Fludioxonil is a synthetic phenylpyrrole-group substance. It is a kind of broad-spectrum, non-systemic fungicide, being used against Fusarium, Rhizoctonia, Alternaria and Botrytis cinerea. For dealing with the fungal diseases, it is usually applied in seed treatment as well as post-harvest treatment of fruits. Fludioxonil is effective in the treatment of many major seed diseases such as seedling blight, stem-base Browning, snow mould and common blunt. For post-harvest treatment, it can deal with Grey mould, storage rot, powdery mildew and black spot. It exerts its effect through interfering with the transport-associated phosphorylation of glucose as well as inhibiting glycerol synthesis, further inhibiting the mycelial growth. When used in combination with thiamethoxam and metalaxyl-M, fludioxonil can also be used for the treatment of pests such as peach-potato aphid, flea beetle and cabbage stem flea beetle.
 | | background | Fludioxonil is a non-systemic fungicide, introduced in 1993 by Ciba-Geigy (now Syngenta). It is used for the treatment of crops (particularly cereals, fruits and vegetables, and ornamental plants; often in combination with another fungicide such as cyprodinil). Brand names include seed treatments: Celest, Agri Star Fludioxonil 41 ST, Dyna-shield Fludioxonil, Maxim 4 FS, and Spirato 480 FS, as well as foliar applications: Switch (fludioxonil + cyprodinil) [1]. Fludioxonil is used against Fusarium, Rhizoctonia, Alternaria and Botrytis cinerea. | | Mechanism of action | Fludioxonil is a new type of pyrrole non-systemic, contact-killing broad-spectrum fungicide. As a seed treatment fungicide, the suspension seed coating agent can control many diseases. The application results show that fludioxonil root irrigation or soil treatment has very good effects on many root diseases such as wilt, root rot, fusarium wilt and vine blight of various crops. In addition, fludioxonil can also be used as a spray to prevent gray mold and sclerotia of various crops. | | effect | Its mode of action is to inhibit transport-associated phosphorylation of glucose, which reduces mycelial growth rate.
| | Indications | It is used for the foliar treatment of wheat, barley, corn, peas, rape, rice, vegetables, grapes, lawns, and ornamental crops. It is used to control Fusarium nivalis, Tilletia rot, Rhizoctonia solani, etc. Special effects; treatment of grain and non-grain seeds, prevention and control of seed and soil-borne pathogens, such as Alternaria, Ascodia, Aspergillus, Fusarium, Bipolaris, Rhizoctonia and Penicillium bacteria. | | Safety | Fludioxonil is safe and harmless to crops at the recommended dose. The seed treatment of Silox is extremely safe for wheat stripe disease, netting disease, hard smut, and snow rot; corn is green and does not affect the emergence of seeds, and can promote the emergence of seeds in advance | | Preparation | Taking nitrophenol as raw material, the intermediate substituted aniline is prepared by etherification, fluorination and reduction, and then reacted with acrylonitrile by diazotization, and finally the ring is closed to obtain fludioxonil. The second preparation method takes substituted benzaldehyde as the starting material and undergoes condensation and cyclization to obtain fludioxonil. The reaction formula is as follows:
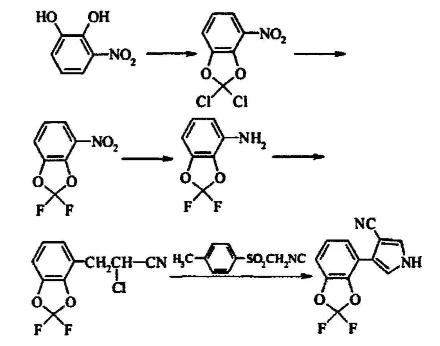 | | References | https://en.wikipedia.org/wiki/Fludioxonil
http://www.agchemaccess.com/Fludioxonil
| | Uses | Fludioxonil is a nonsystemic phenylpyrrole fungicide structurally related to pyrrolnitrin. | | Uses | Agricultural fungicide. | | Uses | Fludioxonil is a non-systemic fungicide used for the seed treatment
control of seedling blight, scab, brown root rot, ear blight, smut and
leaf spots in cereals caused by Fusarium spp., Septuria nodurum, Tilletia
caries, etc. It is also a contact fungicide controlling fruit rot, wood rot, stem
rot and root rot in vines, fruits, field crops, vegetables and rice. | | Definition | ChEBI: A member of the class of benzodioxoles that is 2,2-difluoro-1,3-benzodioxole substituted at position 4 by a 3-cyanopyrrol-4-yl group. A fungicide seed treatment for control of a range of diseases including Fusarium, Rhizoctonia an
Alternaria . | | Metabolic pathway | Limited data are available in the open literature. Information presented in
this summary was abstracted from the data evaluation published by the
Pesticide Safety Directorate (PSD, 1995). Fludioxonil is stable to hydrolytic
and soil degradation. Oxidation and hydration of the cyano moiety
was observed in and on soil surfaces and probably resulted from photolytic
action to yield the corresponding amides and carboxylic acids.
Extensive degradation occurred at the pyrrole ring in plants and animals,
including hydroxylation and oxidation, followed by ring-opening. Modification
of the benzodioxyl moiety was not observed. | | Degradation | Fludioxonil(1) is stable to hydrolytic degradation (up to 30 days at 25 °C)
over the pH range 5-9.
Fludioxonil degraded in pH 7 buffered solution when exposed to UV
light generated by a xenon arc lamp with an estimated DT50 value of 10
days. The chemical nature of three major degradation products (each
accounted ca. 15%) was not determined. |
| | Fludioxonil Preparation Products And Raw materials |
|