- Anandamide
-

- $66.00 / 2mg
-
2024-11-12
- CAS:94421-68-8
- Min. Order:
- Purity: 98.76%
- Supply Ability: 10g
- Anandamide
-

- $66.00 / 2mg
-
2024-11-12
- CAS:94421-68-8
- Min. Order:
- Purity: 98.76%
- Supply Ability: 10g
- Anandamide
-

- $32.00 / 1kg
-
2024-01-08
- CAS:
- Min. Order: 1kg
- Purity: 99.50%
- Supply Ability: 20tons
|
| | ANANDAMIDE Basic information |
| Product Name: | ANANDAMIDE | | Synonyms: | Arachidonylethanolamide,AEA, Anandamide, Arachidonic acid N-(hydroxyethyl)amide;Anandamide (in Tocrisolve 100);Arachidonoyl Ethanolamide Lipid Maps MS Standard;(5Z,8Z,11Z,14Z)-N-(2-Hydroxyethyl)-5,8,11,14-eicosatetraenamide;ARACHIDONYLETHANOLAMIDE, >=97.0% (TL;11,14-eicosatetraenamide(all-z)-n-(2-hydroxyethyl)-8;14-eicosatetraenamide,n-(2-hydroxyethyl)-(all-z)-11;5,8,11,14-eicosatetraenoylethanolamide | | CAS: | 94421-68-8 | | MF: | C22H37NO2 | | MW: | 347.53 | | EINECS: | 200-578-6 | | Product Categories: | Cannabinoid receptor;Cannabinoid | | Mol File: | 94421-68-8.mol | 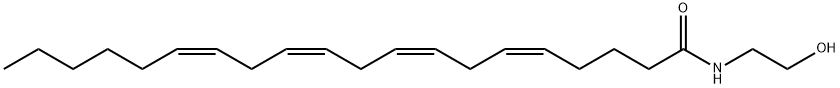 |
| | ANANDAMIDE Chemical Properties |
| Boiling point | 522.3±50.0 °C(Predicted) | | density | 0.92 g/mL at 25 °C (lit.) | | Fp | 14 °C | | storage temp. | -20°C | | solubility | ethanol: soluble | | form | oil | | pka | 14.48±0.10(Predicted) | | color | light yellow | | Water Solubility | 413ug/L(25 ºC) | | Stability: | Stable for 1 year from date of purchase as supplied. Subject to oxidation – protect from air. Solutions in degassed DMSO or ethanol may be stored under nitrogen or argon at -80°C for up to 1 month. |
| Hazard Codes | F | | Risk Statements | 11 | | Safety Statements | 24/25-16-7 | | RIDADR | UN 1170 3/PG 2 | | WGK Germany | 3 | | RTECS | JX3842500 |
| | ANANDAMIDE Usage And Synthesis |
| Description | Anandamide (94421-68-8) is an endogenous cannabinoid receptor agonist.1-3?Ki=89 nM for CB1?and 371 nM for CB2?receptors. | | Uses | Anandamide is an endogenous cannabinoid and TRPV1 receptor agonist. Findings suggest that endocannabinoids such as anandamide may help cause runner’s high, the feeling of euphoria, reduced anxiety and lessened ability to feel pain associated with exercise. | | Definition | ChEBI: An N-(polyunsaturated fatty acyl)ethanolamine resulting from the formal condensation of carboxy group of arachidonic acid with amino group of ethanolamine. | | Hazard | A reproductive hazard. | | Biological Activity | Endogenous cannabinoid and vanilloid receptor agonist, in water-soluble emulsion (for details see TocrisolveTM 100). K i values are 89 and 371 nM for CB 1 and CB 2 receptors respectively; EC 50 values are 18, 31 and 27 nM at GPR55, CB 1 and CB 2 respectively; pK i = 5.68 for rVR1. Also blocks TNF- α -induced NF-kB activation via direct inhibition of IKK. Also available as the pure oil dissolved in ethanol (N-(2-Hydroxyethyl)-5Z,8Z,11Z,14Z-eicosatetraenamide ). | | Biochem/physiol Actions | An arachidonic acid derivative that is an endogenous ligand for the CB cannabinoid receptor and for the VR1 vanilloid receptor. Inhibits calcium currents in neuroblastomas and neurons. Activates the MAP kinase signaling pathway. Inhibits proliferation and induces apoptosis of lymphocytes and human breast cancer cells. | | Enzyme inhibitor | This lipid neurotransmitter and endocannabinoid receptor ligand (FW = 347.54 g/mol; CAS 94421-68-8), also known as arachidonylethanolamide, inhibits calcium ion currents and activates the MAP kinase signaling pathway. Anandamide is a naturally occurring effector of central and peripheral nervous systems, as mediated by CB1 and CB2 cannabinoid receptors, respectively. (These receptors were originally characterized as binding Δ9 -tetrahydrocannabinol, or THC, marijuana’s major psychoactive ingredient.) Anandamide, 2-arachidonoylglycerol, and congeners (N- oleoylethanolamide and N-palmitoylethanolamide) are lipophilic signalling molecules with multiple physiological roles in learning and memory, neuroinflammation, oxidative stress, neuroprotection and neurogenesis. Anandamide likewise plays a role in regulating uterine implantation of the early-stage embryo (blastocyst). Anandamide synthesis occurs from N- acetylphosphatidylethanolamine by multiple pathways that include phospholipase A2, phospholipase C, and NAPE-hydrolyzing phospholipase D (or NAPE-PLD). Target(s): adenylate cyclase; Na + channels; a7-nicotinic acetylcholine receptor; 5 HT3 receptors; Ca 2+ channels; K+ channels, voltage-gated; acylglycerol lipase, or monoacylglycerol lipase. | | storage | Store at -20°C | | References | 1) Devane?et al. (1992),?Isolation and structure of a brain constituent that binds to the cannabinoid receptor; Science,258?1946
2) Devane?et al. (1994),?New dawn of cannabinoid pharmacology; Trends Pharmacol.Sci.,?15?40
3) Hillard?et al. (1997),?Biochemistry and pharmacology of arachidonoylethanolamide, a putative endogenous cannabinoid; J. Lipid Res.,?38?2383 |
| | ANANDAMIDE Preparation Products And Raw materials |
|