- Indacaterol Maleate
-

- $2.00 / 1kg
-
2025-04-02
- CAS:753498-25-8
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 100KG/M
- Indacaterol Maleate
-
- $1.00 / 1kg
-
2025-03-28
- CAS:753498-25-8
- Min. Order: 1kg
- Purity: 99
- Supply Ability: 1
- Indacaterol(Maleate)
-

- $0.00 / 10mg
-
2025-03-04
- CAS:753498-25-8
- Min. Order: 10mg
- Purity: 0.98
- Supply Ability: 10g
|
| | Indacaterol Maleate Basic information |
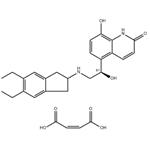
| Product Name: | Indacaterol Maleate | | Synonyms: | 5-[(1R)-2-[(5,6-Diethyl-2,3-dihydro-1H-inden-2-yl)aMino]-1-hydroxyethyl]-8-hydroxy-2(1H)-quinolinone Maleic Acid;Indacaterol Maleic Acid Salt;Onbrez;QAB 149 Maleic Acid;5-[(1R)-2-[(5,6-Diethyl-2,3-dihydro-1H-inden-2-yl)amino]-1-hydroxyethyl]-8-hydroxy-2(1H)-quinolinone (2Z)-2-butenedioate;2(1H)-Quinolinone, 5-[(1R)-2-[(5,6-diethyl-2,3-dihydro-1H-inden-2-yl)aMino]-1-hydroxyethyl] -8-hydroxy-, (2Z)-2-butenedioate (1:1) (salt);2(1H)-Quinolinone, 5-[(1R)-2-[(5,6-diethyl-2,3-dihydro-1H-inden-2-yl)amino]-1-hydroxyethyl]-8-hydroxy-, (2Z)-2-butenedioate (1:1);ndacaterol Maleate | | CAS: | 753498-25-8 | | MF: | C24H28N2O3.C4H4O4 | | MW: | 508.56 | | EINECS: | 691-329-4 | | Product Categories: | Agonist;Amines;Aromatics;Heterocycles;Intermediates & Fine Chemicals;Pharmaceuticals;API | | Mol File: | 753498-25-8.mol | 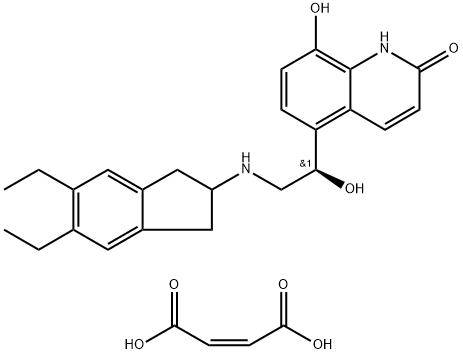 |
| | Indacaterol Maleate Chemical Properties |
| Melting point | 195-197°C (dec.) | | storage temp. | Refrigerator | | solubility | DMSO (Slightly), Methanol (Slightly) | | form | Solid | | color | White to Pale Beige | | InChIKey | IREJFXIHXRZFER-NVEIECOTNA-N | | SMILES | C(/C(=O)O)=C/C(=O)O.[C@H](C1C=CC(O)=C2NC(=O)C=CC=12)(O)CNC1CC2=CC(CC)=C(CC)C=C2C1 |&1:8,r| |
| | Indacaterol Maleate Usage And Synthesis |
| Chemical Properties | White Solid | | Uses | A long-acting β2 adrenoreceptor agonist and bronchodilator, for the treatment of asthma and chronic obstructive pulmonary disease. | | Definition | ChEBI: A maleate salt obtained by reaction of indacaterol with one equivalent of maleic acid. Used for treatment of chronic obstructive pulmonary disease. | | Brand name | Indacaterol is provided as its maleate salt form. It is marketed in Europe as Onbrez and in America as Arcapta Neohaler. | | Mechanism of action | Indacaterol works by stimulating adrenergic beta-2 receptors in the smooth muscle of the airways. This causes relaxation of the muscle, thereby increasing the diameter of the airways, which become constricted in asthma and COPD. It is also long acting due to its high affinity to the lipid raft domains in the airway membrane so it slowly dissociates from the receptors. Indacaterol also has a high intrinsic efficacy so it is also very rapid acting - onset of action occurs within 5 minutes. | | Pharmacology |
Indacaterol maleate, a new, once-daily long-acting beta-agonist (LABA), falls into this category because of its 24-hour duration of action and rapid onset of action. Approved in the EU in 2009 and subsequently approved in several countries worldwide, indacaterol is the first such LABA with both of these properties, which, at least hypothetically, might enhance medication adherence. In cells transfected with stable human b2-adrenoceptors, indacaterol was found to be a nearly full agonist with a maximal production of cAMP (Emax) that was 73 ± 1 (SEM)% of the maximal effect of isoproterenol. Formoterol also displayed close to total b2-adrenoceptor agonist activity with an Emax of 90 ± 1%, whereas salmeterol was only a partial b2-agonist (Emax of 38 ± 1%). Indacaterol also displayed nearly full b2-agonist activity in isolated human bronchi with a maximal relaxant effect of 77 ± 5%. Indacaterol also exhibits functional selectivity for the b2-receptor with only very weak agonist activity at the b1-receptor, similar to the receptor selectivity profile of formoterol. In isolated human bronchi, indacaterol had an onset of action (7.8 ± 0.7 min) similar to that of formoterol and salbutamol and faster than that of salmeterol and a duration of action exceeding 12 h. In the conscious guinea pig, indacaterol administered intratracheally displayed a prolonged (≥ 24 h) protective effect against bronchospasm induced by serotonin, in contrast to much shorter durations of anti bronchoconstrictor activity for salmeterol, formoterol, and salbutamol (12, 4 and 2 h, respectively). Given by nebulization to rhesus monkeys, indacaterol also had the longest duration of bronchoprotective activity (≥ 4.5 h, the last period assessed), as well as inducing the smallest increase in heart rate for the same maximal degree of bronchoconstrictor effect compared with the other b2-agonists, suggesting a cardiovascular safety advantage.
| | Clinical Use | Indacaterol is a b-adrenoceptor agonist currently approved in
Europe as Onbrez, and is marketed by Novartis. It needs to be
taken only once a day, unlike competitors formoterol and salmeterol.
These drugs are used in the treatment of chronic obstructive
pulmonary disease (COPD) and asthma. Onbrez is administered via
an aerosol formulation through a dry powder inhaler. A Phase III
trial published in July 2010 suggested that indacaterol is significantly
more effective than twice-daily formoterol in improving
FEV1 and reduces the need for rescue medication. | | Side effects |
combination of sore throat, runny nose, blocked nose, sneezing; coughing; headache with or without fever; sneezing; muscle pain; muscle spasm; swollen hands, ankles and feet; crushing chest pain (heart problems); neck pain; palpitations (feeling that your heartbeat is unusually fast or irregular); headache; sore throat; cough; runny nose; blocked nose; dry mouth; feeling pressure or pain in the cheeks and forehead (inflammation of the sinuses); chest pain; chest discomfort; pain in muscles, bones or joints; excessive thirst, high urine output, increase appetite with weight loss and tiredness, high level of sugar in the blood (that are signs of a disease called diabetes); tingling and numbness; dizziness vertigo
| | Synthesis | The synthesis
of indacaterol relies on the union of the dihydroindeneamine
region and the quinolinol region of the molecule. Preparation of
the dihydroindene unit of indacaterol was reported by researchers
at Novartis in 2006 and is summarized in the scheme.
2,3-Dihydro-1H-inden-2-amine (59) was protected as its trifluoroacetamide
61 and was followed by Friedel¨CCrafts alkylation with
acetylchloride to give 62. Hydrogenative carbonyl reduction of this
unsymmetrical dihydroindene provided amide 63. An iterative Friedel¨CCrafts acylation/hydrogenation sequence was used to install
the second ethyl group, giving rise to trifluoroacetamide 64.
Basic hydrolysis to remove the trifluoroacetamide functionality,
followed by salt formation by means of gaseous HCl furnished
the dihydroindene amine 65. The synthesis of the remaining
portion of the molecule starts from 8-hydroxyquinoline (66).
Friedel¨CCrafts alkylation with acetylchloride and trichloroaluminum
installed the acetophenone functionality at the 5-position of
the quinoline frame followed by benzyl protection of the hydroxyl
group to give ether 67. Oxidation of quinoline 67 with mCPBA and
acylation of the resulting N-oxide with acetic anhydride and thermal
rearrangement produced quinolone 68. Bromination of the
methyl ketone and subsequent asymmetric reduction provided
(R)-alcohol 69. Bromohydrin 69 was then converted to the epoxide
using potassium carbonate prior to amination of the epoxide with
dihydroindene intermediate 65 to furnish the indacaterol skeleton
70. Hydrogenolytic debenzylation and maleate salt formation
provided indacaterol maleate (XI). 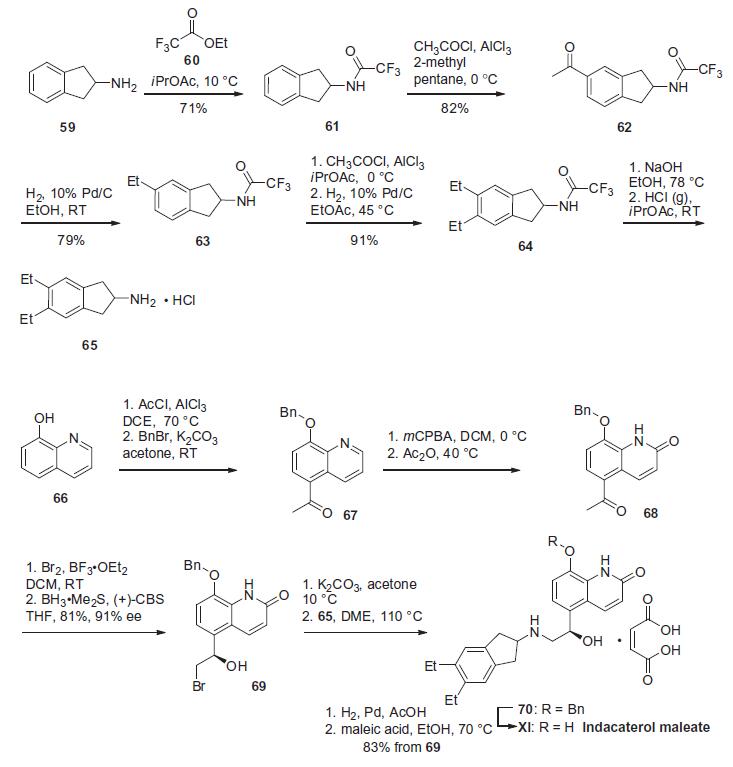
| | Solubility in organics | insoluble in H2O; ≥10.02 mg/mL in ETOH with gentle warming; ≥51.6 mg/mL in DMSO | | References | [1] Tashkin, Donald P. “Indacaterol maleate for the treatment of chronic obstructive pulmonary disease.” Expert Opinion on Pharmacotherapy 11 12 (2010): 2077–85.
|
| | Indacaterol Maleate Preparation Products And Raw materials |
|