Trifluoromethanesulfonic acid difluoromethyl ester manufacturers
|
| | Trifluoromethanesulfonic acid difluoromethyl ester Basic information |
| Product Name: | Trifluoromethanesulfonic acid difluoromethyl ester | | Synonyms: | Trifluoromethanesulfonic acid difluoromethyl ester;Difluoromethyl triflate 95%;Difluoromethyl triflate;Difluoromethyl trifluoromethanesulfonate;Difluoromethyl trifluoromethanesulfonate 95%;Methanesulfonic acid, 1,1,1-trifluoro-, difluoromethyl ester | | CAS: | 1885-46-7 | | MF: | C2HF5O3S | | MW: | 200.08 | | EINECS: | | | Product Categories: | | | Mol File: | 1885-46-7.mol | 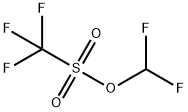 |
| | Trifluoromethanesulfonic acid difluoromethyl ester Chemical Properties |
| Boiling point | 48.5-49 °C | | density | 1.584 g/mL at 25 °C | | Fp | 57℃ | | storage temp. | 2-8°C | | form | liquid | | color | Clear, colourless |
| Hazard Codes | C,Xn | | Risk Statements | 22-34 | | Safety Statements | 26-36/37/39-45 | | RIDADR | UN 2920 3(8) / PGII | | WGK Germany | 3 | | HS Code | 2905190098 |
| | Trifluoromethanesulfonic acid difluoromethyl ester Usage And Synthesis |
| Uses | Difluoromethyl triflate (TfO-CHF2) can be used as a reagent:
- In difluoromethylation reaction.
- To prepare difluoromethoxylated heterocycles by reacting with hydroxylated N-based heterocycles.
- To synthesize trifluoromethylated arenes by treating with diaryliodonium salts in the presence of copper and tetrabutylammonium difluorotriphenylsilicate (TBAT).
It allows for a simple method toward the preparation of difluoromethyl ethers and thioethers under basic conditions from alcohols and thiols. Difluoromethyl phenols can also be obtained in a single pot from boronic acids and C-H activation of arenes. | | General Description | Difluoromethyl triflate (HCF2OTf) is an easy to handle, air-stable and non-ozone-depleting liquid reagent for difluoromethylation. It can be prepared by reacting trifluoromethyltrimethylsilane (TMSCF3) and triflic acid in the presence of titanium tetrachloride (TiCl4). |
| | Trifluoromethanesulfonic acid difluoromethyl ester Preparation Products And Raw materials |
|