- 4-Vinyloxybutanol
-

- $5.00 / 1KG
-
2024-10-11
- CAS:17832-28-9
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 10000kg
|
| | 1,4-Butanediol vinyl ether Basic information |
| | 1,4-Butanediol vinyl ether Chemical Properties |
| Melting point | -33 °C | | Boiling point | 95 °C/20 mmHg (lit.) | | density | 0.939 g/mL at 25 °C (lit.) | | vapor pressure | 0.3 hPa (20 °C) | | refractive index | n20/D 1.444(lit.) | | Fp | 185 °F | | storage temp. | Store at <= 20°C. | | solubility | 75g/l | | form | clear liquid | | pka | 15.04±0.10(Predicted) | | color | Colorless to Light yellow | | Specific Gravity | 0.93 | | explosive limit | 1.4-9.9%(V) | | Water Solubility | 75 g/L (20 ºC) | | LogP | 0.43 at 25℃ | | CAS DataBase Reference | 17832-28-9(CAS DataBase Reference) | | EPA Substance Registry System | 1-Butanol, 4-(ethenyloxy)- (17832-28-9) |
| Hazard Codes | Xn | | Risk Statements | 20/21/22-36/37/38-22-52/53 | | Safety Statements | 26-36-24/25-61 | | RIDADR | NA 1993 / PGIII | | WGK Germany | 1 | | Autoignition Temperature | 225 °C DIN 51794 | | HS Code | 2909 49 80 | | Toxicity | LD50 orally in Rabbit: 1738 mg/kg LD50 dermal Rat > 2000 mg/kg |
| | 1,4-Butanediol vinyl ether Usage And Synthesis |
| Chemical Properties |
1,4-Butanediol vinyl ether (HBVE; 4-Hydroxybutyl vinyl ether) is a colorless or light yellow transparent liquid with a slight odor similar to ether. It is miscible with alcohols, ethers, esters and aromatic hydrocarbons.
| | Synthesis | 1,4-Butanediol vinyl ether is prepared by reacting acetylene and 1,4-butanediol in the presence of potassium hydroxide and potassium alkoxide catalyst: 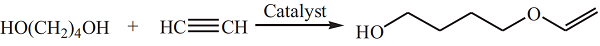 Since 1,4-butanediol contains two hydroxyl groups and the product contains active vinyl ether functional groups, some side reactions will occur. For example, further reaction of HBVE with acetylene to gen�erate divinyl ether, and self-condensation reaction of HBVE to form a cyclic acetal. A superbase catalytic system CsF–NaOH was developed for the preparation of HBVE at a temperature of 138–14 °C and under initial acetylene pressure of 1.0–1.2 MPa. The conversion of 1,4-butanediol was 100% and the total yield of the vinyl ethers was 80% at catalyst dosage (7 mol% CsF and 7 mol% NaOH based on 1,4-butanediol) and after reaction for 3 h. Some researchers also developed heterogeneous catalysts for the reaction of acetylene and 1,4-butanediol to obtain HBVE. Potassium hydroxide was supported on carriers such as aluminum oxide, molecular sieves, silica gel and zirconia.
Since 1,4-butanediol contains two hydroxyl groups and the product contains active vinyl ether functional groups, some side reactions will occur. For example, further reaction of HBVE with acetylene to gen�erate divinyl ether, and self-condensation reaction of HBVE to form a cyclic acetal. A superbase catalytic system CsF–NaOH was developed for the preparation of HBVE at a temperature of 138–14 °C and under initial acetylene pressure of 1.0–1.2 MPa. The conversion of 1,4-butanediol was 100% and the total yield of the vinyl ethers was 80% at catalyst dosage (7 mol% CsF and 7 mol% NaOH based on 1,4-butanediol) and after reaction for 3 h. Some researchers also developed heterogeneous catalysts for the reaction of acetylene and 1,4-butanediol to obtain HBVE. Potassium hydroxide was supported on carriers such as aluminum oxide, molecular sieves, silica gel and zirconia. | | Reactivity Profile |
The structure of the 1,4-Butanediol vinyl ether (HBVE) is a vinyl double bond directly connected with an ether bond, and due to the influence of an adjacent oxygen atom, the double bond is an electron-rich double bond and shows higher reactivity. HBVE also has a hydroxyl, and can react with a series of resins. It has wide application in fluororesin and coatings.
| | Flammability and Explosibility | Flammable |
| | 1,4-Butanediol vinyl ether Preparation Products And Raw materials |
|