|
|
| | 3-Chloropropionyl chloride Basic information |
| | 3-Chloropropionyl chloride Chemical Properties |
| Melting point | -32 °C | | Boiling point | 143-145 °C(lit.) | | density | 1.33 g/mL at 25 °C(lit.) | | vapor pressure | 10.0 hPa | | refractive index | n20/D 1.457(lit.) | | RTECS | UC3934000 | | Fp | 146 °F | | storage temp. | Flammables area | | solubility | Chloroform, Ethyl Acetate | | form | liquid | | color | red-brown | | PH | <7 (H2O) | | explosive limit | 8.8-20.2%(V) | | Water Solubility | reacts | | Sensitive | Moisture Sensitive | | BRN | 635814 | | Stability: | Moisture Sensitive | | InChIKey | INUNLMUAPJVRME-UHFFFAOYSA-N | | CAS DataBase Reference | 625-36-5(CAS DataBase Reference) | | NIST Chemistry Reference | Propanoyl chloride, 3-chloro-(625-36-5) | | EPA Substance Registry System | Propanoyl chloride, 3-chloro- (625-36-5) |
| | 3-Chloropropionyl chloride Usage And Synthesis |
| Description | 3-Chloropropionyl chloride is an important bifunctional reagent. It is capable of acylation and possesses a 2-chloro-ethyl fragment (CH2CH2Cl), which can be subjected to nucleophilic substitution and serves as a masked vinyl group. It can be used as a starting material in many reactions to construct a variety of (hetero)cyclic compounds. | | Chemical Properties | Clear colorless to dark brown liquid. Soluble in ethanol, ether and chloroform. Slightly soluble in water. | | Uses | 3-Chloropropionyl chloride is a reagent used in the synthesis of Beclamide (B119400), which is a chlorinated benzylpropanamide used as an anticonvulsant drug. It is used in the treatment of tonic-clonic seizyres and has sedative properties. | | Preparation | 3-Chloropropionyl chloride (1) is commercially available and can be prepared from β-propiolactone (2) and thionyl chloride.1 Other standard methods available for the preparation of acyl chlorides can also be applied: the reaction of acrylic acid (3) or 3-chloropropionic acid (4) with thionyl chloride, phosphoryl chloride, phosgene, or phosphorus trichloride.
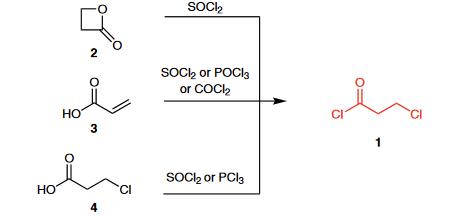 | | Reactions | (1) The Friedel¨CCrafts acylation of tert-butylbenzene (5) with 3-chloropropionyl chloride (1) followed by cyclization provid- ed indanone 6, which was further transformed into urea derivative 7, a potent TRPV1 antagonist.
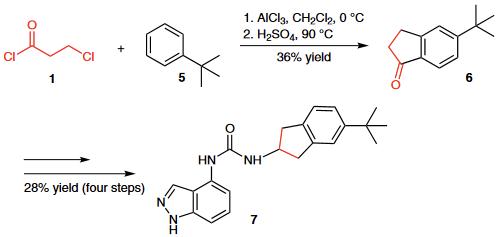
(2) A novel high-yielding one-pot microwave-assisted synthesis of condensed 5-substituted pyranoisoquinoline-1,6-diones 9 from 2-substituted isoquinoline-1,3-diones 8 and 3-chloropropionyl chloride (1) was reported.
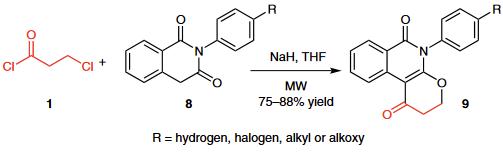
(3) Acylation of 2-aminophenol (12) with 3-chloropropionyl chloride (1), followed by cyclization in the presence of polyphosphoric acid (PPA), gave benzoxazole 13, which was further reacted with 4-chlorophenyl-1-piperazine to yield the target benzo[d]oxazole analogue 14, a selective dopamine D4 receptor ligand. | | Flammability and Explosibility | Non flammable | | Synthesis | 3-Chloropropionyl chloride is produced by reaction of acrylic acid with hydrogen chloride and phosgene in the presence of, for example,dimethylformamide as catalyst, or by reaction of propiolactone with thionyl chloride. |
| | 3-Chloropropionyl chloride Preparation Products And Raw materials |
|