- sodium chlorite
-

- $600.00 / 1T
-
2024-10-25
- CAS:7758-19-2
- Min. Order: 1T
- Purity: 99%
- Supply Ability: 20tons
- Sodium chlorite;Naclo2
-

- $3.00 / 50KG
-
2024-10-25
- CAS:7758-19-2
- Min. Order: 1T
- Purity: 25%;31%;78%;80%;82%;90%
- Supply Ability: 1000tons/month
- Sodium chlorite
-

- $1.00 / 1kg
-
2024-10-25
- CAS:7758-19-2
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 10 mt
Related articles - What is sodium chlorite used for?
- Sodium chlorite is a very efficient bleaching agent. Its oxidation potential allows a controlled bleaching that is not attaina....
- Mar 16,2024
|
| Product Name: | Sodium chlorite | | Synonyms: | SodiuM chlorite, unstabilized, pure, 80% 500GR;Scentrex? DTS 1.05 sachet;Sodium chlorite puriss. p.a., 80% (RT);Sodium chlorite technical grade, 80%;Sodium chlorite, technical, 80%;Sodium chlorite, Unstabilized;Sodium Chlorite, Anhydrous;TEXTONE | | CAS: | 7758-19-2 | | MF: | ClNaO2 | | MW: | 90.44 | | EINECS: | 231-836-6 | | Product Categories: | Water Ttreatment Chemicals;Inorganic Salts;Synthetic Reagents;Analytical Reagents for General Use;Puriss p.a.;Q-S, Puriss p.a.;Essential Chemicals;Routine Reagents;Technical Grade;Water treatment;7758-19-2 | | Mol File: | 7758-19-2.mol | 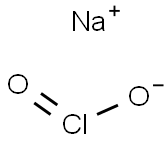 |
| | Sodium chlorite Chemical Properties |
| Melting point | 190 °C (dec.) | | density | 2.5 g/cm3 | | vapor pressure | 0Pa at 25℃ | | solubility | Methanol (Slightly), Water (Slightly) | | form | Powder | | color | White | | Odor | odorless | | PH Range | 10 - 11 | | explosive limit | 7% | | Water Solubility | 39 g/100 mL (17 ºC) | | Sensitive | Hygroscopic | | Merck | 14,8600 | | Dielectric constant | 6.1(Ambient) | | Stability: | Stable. Incompatible with phosphorus, sulphur, zinc, ammonia, finely powdered metals, strong reducing agents, acids, organic materials. | | LogP | -2.7 at 25℃ | | CAS DataBase Reference | 7758-19-2(CAS DataBase Reference) | | IARC | 3 (Vol. 52) 1991 | | EPA Substance Registry System | Sodium chlorite (7758-19-2) |
| | Sodium chlorite Usage And Synthesis |
| Description | Sodium chlorite (NaClO2) is a white crystalline solid that can accelerate the burning of organic substances. It is widely used in various industries, including paper manufacturing, water purification, wood pulp bleaching, textiles, fats and oils, as a disinfectant, and in many other fields. In organic synthesis, sodium chlorite is often used to convert aldehydes to carboxylic acids and alkyl furans to 4-oxo-2-alkenoic acids. Moreover, when combined with an acid such as citric acid, acidified sodium chlorite can be used to sanitize surfaces and rinse a variety of foods. In the military, sodium chlorite can be used to combat contaminants such as harmless microbes and food pathogens.
 | | Uses | Sodium chlorite is a kind of efficient bleacher and bactericide. It is used to bleach various fibers including cotton, linen, mulberry, reed, viscose fiber and so on. Additionally, it can bleach sugar, flour, starch, ointment, wax, oil, and more. Sodium chlorite also serves as a leather depilator, a surface treatment for certain metals, and a water and sewage treatment solution. It can even purify trace amounts of nitric oxide in coke oven gas. | | Production | The industrial production includes hydrogen peroxide method and electrolysis method.
For hydrogen peroxide method, we dissolve sodium chlorate in water to obtain the solution containing 250 g/L sodium chlorate, add chlorine dioxide generator and then adjust sulfuric acid into 4mol/L H2SO4. Add the gas mixture containing sulfur dioxide and air mixture and 4mol/L H2S04 into the chlorine dioxide generator to react, pass the chlorine dioxide gas generated through 3 bubbling absorption towers in series and obtain the solution containing 140 ~ 160 g/L NaClO2 after the reaction with 27.5% hydrogen peroxide and 18% ~ 20% liquid caustic soda. The clear liquor after the precipitation is the liquid sodium chlorite product. Control the concentration of sodium chlorite solution between 350g/L and 400g/L by evaporation and concentration and then obtain the finished product of solid sodium chlorite after cooling crystallization, filtration and drying. As follows:
2NaClO3+SO2+H2SO4→2C1O2+2NaHSO4
2C1O2+2NaOH+H2O2→2NaC1O2+2H2O+O2
For electrolysis method, we dissolve sodium chlorate in mixed acid composed by water and sulfuric acid with 260g sodium chlorate in per milliliter, add the mixed acid into chlorine dioxide generator to react with the mixed gas made up of sulfur dioxide and air (containing 8% ~ 10% sulfur dioxide), take 15% chlorine dioxide gas generated into the cathode chamber of electrolytic bath and add brine and distilled the water continuously in the anode chamber to carry out electrolysis to generate the sodium chlorite solution containing about 20% sodium chlorite. We can obtain the finished product of solid sodium chlorite after removing the trace of free chlorine dioxide and spray-drying at 130℃, or by evaporation concentration, cooling crystallization, filtration and drying. As follows:
2NaClO3+H2SO4+SO2→2ClO2+2NaHSO4
C1O2+e→C1O2-
2Cl-+2e→C12↑
Na++ClO2-→NaClO2
| | Identification Test | The sodium test (IT-28) is positive.
With the addition of dilute hydrochloric acid in the 5% sample liquid, yellow gas should be produced and the liquid becomes yellow-brown.
The litmus red test paper turns blue after soaking up with 5% sample liquid infiltration.
| | Content Analysis | Weigh accurately 100 mg of the sample and dissolve it in water to make 250ml. Take 20ml of its solution in the iodine flask, add 12ml of 1mol/L of sulfuric acid and 25ml of potassium iodide test solution (TS-192), plug tightly at once, keep it in the dark for 5min and then add 0.5ml of starch test solution (TS-235). Use 0.1mol/L sodium thiosulfate solution to titrate and carry out the blank test at the same time. Per milliliter of 0.1mol/L sodium thiosulfate solution acts as 2.261mg sodium chlorite (NaClO2).
| | Toxicity | LD50166mg/kg (rats, per os).
Chlorine dioxide can be produced in use and staying in the air containing 45mg/kg chlorine dioxide for several hours can lead to death for a groundhog. It is irritating to respiratory mucous and eyes.
| | References | https://pubchem.ncbi.nlm.nih.gov/compound/sodium_chlorite#section=Top
https://en.wikipedia.org/wiki/Sodium_chlorite
| | Chemical Properties | It is a white crystalline powder or flakes that is readily soluble in water. It is slightly hygroscopic. | | Uses | Sodium chlorite may be used in the synthesis of chlorine dioxide and as a hydroxylating agent for the hydroxylation of androstenedione (steroid). | | Uses | Sodium chlorite is used in the in situ generation of chlorine dioxide for stripping of textiles, bleaching, pulp and paper industries. It acts as a disinfectant in water treatment plant and as a preservative in eye drops. It is also used in contact lens cleaning solution and for sanitizing air ducts. It is associated with zinc chloride and used as a component in therapeutic rinses, toothpastes, mouth sprays and gels. It is utilized for the synthesis of 4-oxo-2-alkenoic acids from alkyl furans. Further, it is involved as a reagent in Pinnick oxidation reaction to prepare carboxylic acid from aldehydes. | | Definition | ChEBI: Sodium chlorite is an inorganic sodium salt in which chlorite is the counterion. It has a role as an oxidising agent. It is an inorganic sodium salt and a chlorite salt. | | General Description | Sodium chlorite appears as a white crystalline solid. Difficult to burn, but accelerates the burning of organic substances. Forms explosive mixtures with certain combustible materials. May explode under prolonged exposure to heat or fire. Used in water purification, to bleach wood pulp, textile, fats, oils; and for many other uses. | | Air & Water Reactions | Soluble in water. | | Reactivity Profile | SODIUM CHLORITE SOLUTION is an oxidizing agent. Can react with acids to form spontaneously explosive chlorine dioxide gas (ClO2). Reacts with ammonia to produce ammonium chlorite, which is shock-sensitive. Finely divided metallic or organic substances in dry mixture with chlorites are highly flammable and may be ignited on friction (Lab. Gov. Chemist 1965). A mixture of organic matter and solid sodium chlorite can be extremely sensitive to heat, impact, or friction (Diox Process 1949). Sodium chlorite reacts very violently with organic materials containing divalent sulfur or with free sulfur (may ignite). | | Hazard | Flammable, strong oxidizing agent, dan-
gerous fire and moderate explosion risk. (Solution)
Strong irritant to skin and tissue. | | Health Hazard | TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution. | | Fire Hazard | Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated. | | Agricultural Uses | Chlorite is a group of greenish clay minerals of variable composition (similar to mica in structure), which crystallizes in the monoclinic system. The term chlorite is derived from 'chloros', the Greek word for green.
Chlorites are composed of complex silicates of aluminum, magnesium and iron in combination with water.
These are often called 2:2 type clays because they are similar to the unit lattice of vermiculite. But strictly speaking, they are 2:1:1 type clays. A layer of chlorite has 2 silicate tetrahedral units, one alumina octahedral unit and one magnesium octahedral sheet. It has a low cation exchange capacity. Chlorites are most commonly found in low-grade metamorphic rocks. They also occur as secondary minerals in igneous rocks as alteration products of pyroxenes, amphiboles and micas.
Chlorites are infrequent in soils and when present, make up a small fraction of clay minerals. Chlorites are primary minerals and form vermiculites and smectites. Chlorites do not swell on wetting. | | Safety Profile | Poison by ingestion. An experimental teratogen. Experimental reproductive effects. Questionable carcinogen with experimental carcinogenic data. Mutation data reported. May act as an irritant due to its oxidizing power. A powerful oxidzing agent; ignited by friction, heat, or shock. An explosive sensitive to impact or heating to 200'. Potentially explosive reaction with acids, oils, organic matter, oxahc acid + water, zinc. Violent reaction or iption with carbon (above 60'), ethylene glycol (at loo'), phosphorus (above SO0), sodum dithionate, sulfur-containing materials. Can react vigorously on contact with reducing materials. When heated to decomposition it emits highly toxic fumes of Cland NazO. Used as a bleachmg agent. See also CHLORITES. | | Purification Methods | Crystallise the chlorite from hot water and store it in a cool place. It has also been crystallised from MeOH by counter-current extraction with liquid ammonia [Curti & Locchi Anal Chem 29 534 1957]. A major impurity is chloride ion which can be removed by recrystallisation from 0.001M NaOH. [Schmeisser in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I p 312 1963.] |
| | Sodium chlorite Preparation Products And Raw materials |
|