- (R)-(+)-Bay-K-8644
-

- $52.00 / 1mg
-
2024-11-19
- CAS:98791-67-4
- Min. Order:
- Purity: 96.51%
- Supply Ability: 10g
|
| | R(+)-BAY K 8644 Basic information |
| Product Name: | R(+)-BAY K 8644 | | Synonyms: | (4R)-1,4-DIHYDRO-2,6-DIMETHYL-5-NITRO-4-[(2-TRIFLUOROMETHYL)PHENYL]-3-PYRIDINECARBOXYLIC ACID METHYL ESTER;R-(+)-1,4-DIHYDRO-2,6-DIMETHY-5-NITRO-(2-[TRIFLUOROMETHYL]-PHENYL)-3-PYRIDINE CARBOXYLIC ACID METHYL ESTER;R(+)-BAY K 8644;R(+)-BAY K 8644 CA2(+) CHANNEL BLOCKE;(+/-)-BAY K 8644, 0 TO 5 °C;R(+)-BAY K 8644 98% 0 C;(4R)-1,4-Dihydro-2,6-dimethyl-5-nitro-4-(2-trifluoromethyl)phenyl)-3-pyridinecarboxylicacidmethylester;r-(+)-1,4-dihydro-2,6-dimethyl-5-nitro-4-[2-(trifluoromethyl)phenyl]-3-pyridinecarboxylic acid methyl ester | | CAS: | 98791-67-4 | | MF: | C16H15F3N2O4 | | MW: | 356.3 | | EINECS: | | | Product Categories: | Calcium channel | | Mol File: | 98791-67-4.mol | 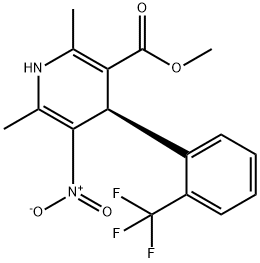 |
| | R(+)-BAY K 8644 Chemical Properties |
| storage temp. | 2-8°C | | solubility | H2O: slightly soluble | | form | solid | | color | yellow |
| Hazard Codes | Xi | | Risk Statements | 36/38 | | Safety Statements | 26-36 | | WGK Germany | 3 |
| | R(+)-BAY K 8644 Usage And Synthesis |
| Description | BAY-K-8644, originally described as a modulator of potential operated calcium channels, exists as two enantiomers that have opposite actions. (R)-(+)-BAY-K-8644 is an L-type channel blocker that has negative inotropic and vasodilatory effects at 1 μM. Intracerebroventricular administration of this enantiomer has no effect on motor function in mice, whereas (S)-(−)-BAY-K-8644 impairs rotarod and motor activity, an effect that is blocked by (R)-(+)-BAY-K-8644. | | Uses | (R)-(+)-Bay K 8644 is a Ca2+ channel inhibitor, whose enantiomer is a Ca2+ channel activator. | | Definition | ChEBI: (R)-Bay-K-8644 is a methyl 2,6-dimethyl-5-nitro-4-[2-(trifluoromethyl)phenyl]-1,4-dihydropyridine-3-carboxylate in which the 4-position has (R)-configuration. It is an enantiomer of a (S)-Bay-K-8644. | | Biological Activity | L-type Ca 2+ -channel blocker with negative inotropic and vasodilatatory effects in vivo . Enantiomer showing opposite effects to the racemate (1,4-Dihydro-2,6-dimethyl-5-nitro-4-[2-(trifluoromethyl)phenyl]-3-pyridinecarboxylic acid, methyl ester ) and (S)-(-)- enantiomer ((4S)-1,4-Dihydro-2,6-dimethyl-5-nitro-4-[2-trifluoromethyl)phenyl]-3-pyridinecarboxylic acid methyl ester ). In combination with BIX-01294, helps generate induced pluripotent stem cells (iPSCs) from mouse embryonic fibroblasts (MEFs). | | storage | Store at +4°C |
| | R(+)-BAY K 8644 Preparation Products And Raw materials |
|