|
|
| | 2,3,4-Trimethoxybenzaldehyde Basic information |
| Product Name: | 2,3,4-Trimethoxybenzaldehyde | | Synonyms: | 2,3,4-trimethoxy-benzaldehyd;2,3,4-TRIMETHOXYBENZALDEHYDE FOR SYNTHES;Trimetazidine Impurity C;2,3,4-TriMethoxybenzaldehyde, 97+%;2,3,4 TRIMETHOXYBENZYLALDEHYDE;2,3,4-TRIMETHOXYBENZALDEHYDE;2,3,4-TMB;2,3,4-Trimethoxybenzaldehyde, 99+% | | CAS: | 2103-57-3 | | MF: | C10H12O4 | | MW: | 196.2 | | EINECS: | 218-271-0 | | Product Categories: | Aromatics, Impurities, Intermediates;BUILDING BLOCKS;Benzaldehyde;API intermediates;(intermediate of trimetazidine.);Aldehydes;C10 to C21;Carbonyl Compounds;Aromatic Aldehydes & Derivatives (substituted);bc0001;2103-57-3 | | Mol File: | 2103-57-3.mol | 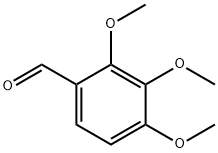 |
| | 2,3,4-Trimethoxybenzaldehyde Chemical Properties |
| Melting point | 38-40 °C(lit.) | | Boiling point | 168-170 °C12 mm Hg(lit.) | | density | 1.2166 (rough estimate) | | refractive index | n20/D 1.5547(lit.) | | Fp | >230 °F | | storage temp. | Inert atmosphere,2-8°C | | solubility | methanol: 0.1 g/mL, clear | | form | Crystals or Crystalline Powder | | color | White to light yellow | | Sensitive | Air Sensitive | | BRN | 981091 | | InChI | InChI=1S/C10H12O4/c1-12-8-5-4-7(6-11)9(13-2)10(8)14-3/h4-6H,1-3H3 | | InChIKey | UCTUXUGXIFRVGX-UHFFFAOYSA-N | | SMILES | C(=O)C1=CC=C(OC)C(OC)=C1OC | | CAS DataBase Reference | 2103-57-3(CAS DataBase Reference) | | NIST Chemistry Reference | Benzaldehyde, 2,3,4-trimethoxy-(2103-57-3) | | EPA Substance Registry System | Benzaldehyde, 2,3,4-trimethoxy- (2103-57-3) |
| | 2,3,4-Trimethoxybenzaldehyde Usage And Synthesis |
| Description |
2,3,4-Trimethoxybenzaldehyde is a white crystalline powder, and the chemical formula is C 10H 12O 4; the molecular weight is 196.2; the fusing point is 38-40 degrees, and is a kind of important medicine intermediate; it is mainly used in new type drug Ca2+ Synthesizing of channel blocker. The alternative cerebral arteries that are used for this medicine reduce the headache incidence, mainly for the preparation of the intermediate of trimetazidine.
| | Synthesis | A coking gallic acid is utilized as a raw material, and dimethyl sulfate is utilized as an alkylate reagent; under the condition of the existence of sodium hydroxide, methylation is performed through an O-alkylation reaction to obtain an intermediate 1, 2, 3-trimethoxybenzene. After that, the 1,2, 3-trimethoxybenzene and a Vilsmeier-Haack reagent are subjected to a formylation reaction to get the 2,3,4-Trimethoxybenzaldehyde. | | Chemical Properties | white to light yellow crystals or cryst. powder | | Uses | 2,3,4-Trimethoxybenzaldehyde was used to study the effects of 2,3,4-trimethoxybenzaldehyde on tubulin-dependent GTP hydrolysis. | | Uses | 2,3,4-Trimethoxybenzaldehyde is a trimethoxylated aromatic aldehyde. 2,3,4-Trimethoxybenzaldehyde is an Impurity of the anti-anginal drug Trimetazidine (T795610). |
| | 2,3,4-Trimethoxybenzaldehyde Preparation Products And Raw materials |
| Raw materials | Gallic acid-->Benzenemethanamine, 2,3,4-trimethoxy-N,N-dimethyl--->2,3,4-TRIMETHOXYBENZYL ALCOHOL-->2,3,4-Trihydroxybenzaldehyde-->1,2,3-Trimethoxybenzene | | Preparation Products | Trimethoprim-->Phenethyl alcohol-->2,3-Dimethoxy-5-methyl-p-benzoquinone-->2,3,4-TRIMETHOXYPHENYLACETIC ACID-->Trimetazidine-->trans-2,3,4-Trimethoxycinnamic acid-->Lomerizine hydrochloride-->(2E)-1-(3-nitrophenyl)-3-(2,3,4-trimethoxyphenyl)prop-2-en-1-one-->1-methyl-3,5-bis(2,3,4-trimethoxybenzylidene)-4-piperidinone-->1,2,3,4-tetramethoxy-5-allylbenzene |
|