- NADP
-

- $10.00 / 25kg
-
2025-04-23
- CAS:53-59-8
- Min. Order: 1kg
- Purity: 99.5%
- Supply Ability: 100
|
| | Triphosphopyridine nucleotide Basic information |
| Product Name: | Triphosphopyridine nucleotide | | Synonyms: | B-NICOTINAMIDEADENINEDINUCLEOTIDEPHOSPHATE,HYDRATE;TRIPHOSPHOPYRIDINE NUCLEOTIDE;CODEHYDROGENASE II;BETA-NICOTINAMIDE ADENINE DINUCLEOTIDE PHOSPHORIC ACID;BETA-NICOTINAMIDE ADENINE DINUCLEOTIDE PHOSPHATE REDUCED;BETA-NADP;β-Nicotinamide adenine dinucleotide phosphate (NADP);BETA-NICOTINAMIDE ADENINE DINUCLEOTIDE PHOSPHATE
(NADP+) | | CAS: | 53-59-8 | | MF: | C21H28N7O17P3 | | MW: | 743.41 | | EINECS: | 200-178-1 | | Product Categories: | 53-59-8 | | Mol File: | 53-59-8.mol | 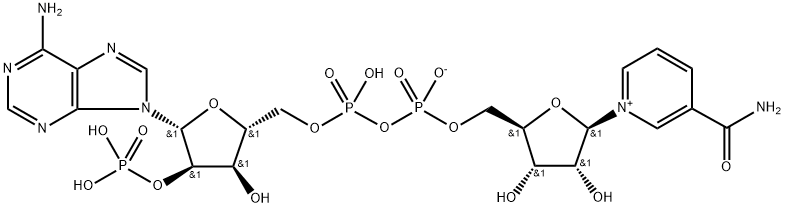 |
| | Triphosphopyridine nucleotide Chemical Properties |
| storage temp. | Keep in dark place,Inert atmosphere,Store in freezer, under -20°C | | solubility | H2O: 50 mg/mL, clear, slightly yellow | | form | powder to crystal | | pka | pKa1 3.9; pKa2 6.1(at 25℃) | | color | White to Orange to Green | | Water Solubility | H2O: soluble 50mg/mL, clear, colorless to faintly yellow | | λmax | 260nm(lit.) | | Merck | 14,6348 | | InChIKey | XJLXINKUBYWONI-WFYQBQJJNA-N | | EPA Substance Registry System | Adenosine 5'-(trihydrogen diphosphate), 2'-(dihydrogen phosphate), P'.fwdarw.5'-ester with 3-(aminocarbonyl)-1-.beta.-D-ribofuranosylpyridinium, inner salt (53-59-8) |
| Hazard Codes | Xi | | Risk Statements | 36/37/38 | | Safety Statements | 26-36 | | WGK Germany | 3 | | RTECS | UU3440000 | | F | 10-21 | | HS Code | 2934.99.9001 |
| | Triphosphopyridine nucleotide Usage And Synthesis |
| Description | Triphosphopyridine nucleotide is a coenzyme composed of ribosylnicotinamide 5'-phosphate (NMN) coupled by pyrophosphate linkage to the 5'-phosphate adenosine 2',5'-bisphosphate. Triphosphopyridine nucleotide serves as an electron carrier in a number of reactions, being alternately oxidized (NADP+) and reduced (NADPH). | | Chemical Properties | Triphosphopyridine nucleotide is white or off-white powder, it is easy to absorb moisture and deliquescence. pKa{1}=3.9; pKa{2}=6.1. It is soluble in water, methanol, insoluble in ethanol, insoluble in ether and ethyl acetate. | | Uses | β-Nicotinamide adenine dinucleotide phosphate hydrate is suitable for use in:
the measurement of Glucose-6-phosphate dehydrogenase activity
the Cytochrome P450 3A4 assay as a part of NADPH-regenerating system
the Cytochrome P450 2D6 assay as a part of NADPH-regenerating system
the determination of Glucose-6-phosphate content | | Definition | The oxidized form of nicotinamide adenine dinucleotide phosphate (NADP) that receives electrons
from photosystem I during photosynthesis. It exists
as an anion under normal physiologic conditions. | | Biological Functions | Triphosphopyridine nucleotide (NADP) serves as an electron carrier in a number of reactions, being alternately oxidized (NADP+) and reduced (NADPH). | | Biochem/physiol Actions | β-Nicotinamide adenine dinucleotide 2′-phosphate (NADP+) and β-Nicotinamide adenine dinucleotide 2′-phosphate, reduced (NADPH) comprise a coenzyme redox pair (NADP+:NADPH) involved in a wide range of enzyme catalyzed oxidation reduction reactions. The NADP+/NADPH redox pair facilitates electron transfer in anabolic reactions such as lipid and cholesterol biosynthesis and fatty acyl chain elongation. The NADP+/NADPH redox pair is used in a variety of antioxidation mechanism where it protects agains reactive oxidation species accumulation. NADPH is generated in vivio by the pentose phosphate pathway (PPP). | | Synthesis | Triphosphopyridine nucleotide is prepared by the reaction of NADPH. It is synthesised mainly by the interaction of both NfrA1 enzyme and a Bacillus subtilis under the conditions of bacterial luciferase. Reaction conditions were as follows: with hydrogenchloride; NfrA1 enzyme; nitrofurazone; 2-amino-2-hydroxymethyl-1,3-propanediol In water at 23℃; pH=7.0; Enzyme kinetics; Further Variations; Reagents; Oxidation.
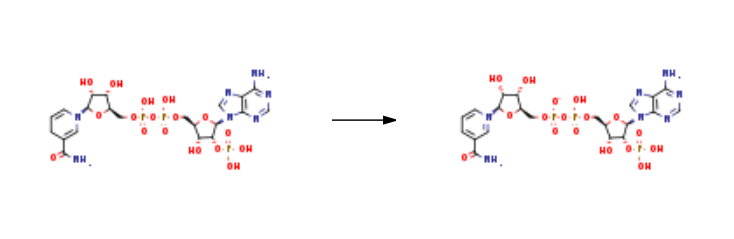 | | Purification Methods | Purify NMN by passage through a column of Dowex-1 (Clform) and washing with H2O until no absorbance is observed at 260 nm. The tubes containing NMN are pooled, adjusted to pH 5.5-6 and evaporated in vacuo to a small volume. This is adjusted to pH 3 with dilute HNO3 in an ice-bath and treated with 20volumes of Me2CO at 0-5o. The heavy white precipitate is collected by centrifugation at 0o. It is best stored wet and frozen or it can be dried to give a gummy residue. It has max 266nm ( 4,600) and min 249nm ( 3600) at pH 7.0 (i.e. no absorption at 340nm). It can be estimated by reaction with CNor hydrosulfite which form the 4-adducts (equivalent to NADH) which have UV max 340nm ( 6,200). Thus after reaction, an OD340 of one is obtained from a 0.1612mM solution in a 1cm path cuvette. [Plaut & Plaut Biochemical Preparations 5 56 1957, Maplan & Stolzenbach Methods Enzymol 3 899 1957, Kaplan et al. J Am Chem Soc 77 815 1955, Beilstein 22/2 V 168.] |
| | Triphosphopyridine nucleotide Preparation Products And Raw materials |
|